20300-02-1
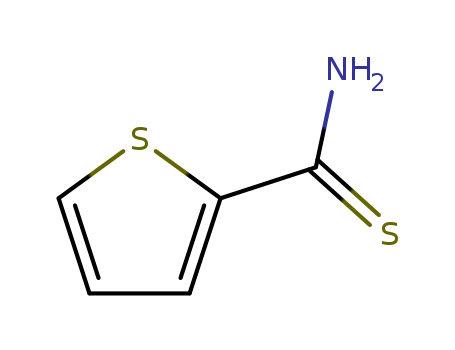
- Product Name:Thiophene-2-carbothioamide
- Molecular Formula:C5H5NS2
- Purity:99%
- Molecular Weight:143.233
Product Details;
CasNo: 20300-02-1
Molecular Formula: C5H5NS2
factory and supplier 20300-02-1 Thiophene-2-carbothioamide in stock
- Molecular Formula:C5H5NS2
- Molecular Weight:143.233
- Vapor Pressure:0.0129mmHg at 25°C
- Melting Point:106 °C
- Refractive Index:1.701
- Boiling Point:259.5 °C at 760 mmHg
- PKA:12.56±0.29(Predicted)
- Flash Point:110.7 °C
- PSA:86.35000
- Density:1.357 g/cm3
- LogP:2.08260
Thiophene-2-thiocarboxamide (Cas 20300-02-1) Usage
InChI:InChI=1/C5H5NS2/c6-5(7)4-2-1-3-8-4/h1-3H,(H2,6,7)
20300-02-1 Relevant articles
A practical synthesis of 3,4-diethoxybenzthioamide based on Friedel-Crafts reaction with potassium thiocyanate in methanesulfonic acid.
Aki, Shinji,Fujioka, Takafumi,Ishigami, Masashi,Minamikawa, Jun-ichi
, p. 2317 - 2320 (2002)
The synthesis of 3,4-diethoxybenzthioami...
An efficient and convenient route for the synthesis of thiophene-2-carboxamidines as potential inhibitors of nitric oxide synthase (NOS)
Rashid,Singh, Dharmendra,Sanjayan, Gangadhar J.
, (2019)
A mild and efficient synthesis of substi...
Aerobic Visible-Light Induced Intermolecular S?N Bond Construction: Synthesis of 1,2,4-Thiadiazoles from Thioamides under Photosensitizer-Free Conditions
Wang, Hui,Xie, Shihua,Zhu, Hongjun,Zhuo, Liang
supporting information, p. 3398 - 3402 (2021/06/25)
Aerobic visible-light induced intermolec...
Mo (CO)6-assisted Pd-supported magnetic graphene oxide-catalyzed carbonylation-cyclization as an efficient way for the synthesis of 4(3H)-quinazolinones
Bahadorikhalili, Saeed,Ansari, Samira,Hamedifar, Haleh,Ma'mani, Leila,Babaei, Mohsen,Eqra, Rahim,Mahdavi, Mohammad
, (2019/02/14)
In this paper, a novel catalyst is intro...
Iminothioethers as Hydrogen Sulfide Donors: From the Gasotransmitter Release to the Vascular Effects
Barresi, Elisabetta,Nesi, Giulia,Citi, Valentina,Piragine, Eugenia,Piano, Ilaria,Taliani, Sabrina,Da Settimo, Federico,Rapposelli, Simona,Testai, Lara,Breschi, Maria Cristina,Gargini, Claudia,Calderone, Vincenzo,Martelli, Alma
, p. 7512 - 7523 (2017/09/23)
The gasotransmitter hydrogen sulfide (H2...
20300-02-1 Process route
-
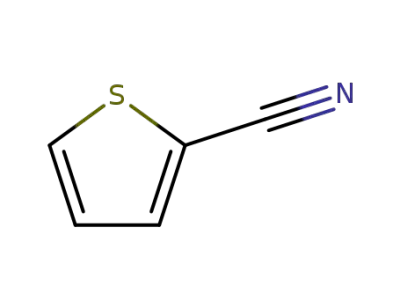
-
1003-31-2
thiophene-2-carbonitrile

-
-
20300-02-1
thiophene-2-carbothioamide
| Conditions | Yield |
|---|---|
|
With
sodium hydrogensulfide; magnesium chloride;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 1h;
|
90% |
|
With
hydrogen sulfide;
In
dimethyl sulfoxide;
at 20 ℃;
|
80% |
|
With
ethanol; ammonia;
anschliessend Behandeln mit Schwefelwasserstoff;
|
|
|
With
pyridine; hydrogen sulfide; triethylamine;
|
|
|
With
O,O-Diethyl hydrogen phosphorodithioate;
In
tetrahydrofuran;
at 66 ℃;
for 5h;
Conversion of starting material;
Heating / reflux;
|
|
|
With
chloroacetonitrile; O,O-Diethyl hydrogen phosphorodithioate;
In
tetrahydrofuran;
Conversion of starting material;
Heating / reflux;
|
|
|
With
O,O-Diethyl hydrogen phosphorodithioate; trichloroacetonitrile;
In
tetrahydrofuran;
Conversion of starting material;
Heating / reflux;
|
|
|
With
O,O-Diethyl hydrogen phosphorodithioate; acetonitrile;
Conversion of starting material;
Heating / reflux;
Neat (no solvent);
|
|
|
With
O,O-Diethyl hydrogen phosphorodithioate; acetonitrile;
In
tetrahydrofuran;
at 66 ℃;
for 5h;
Conversion of starting material;
Heating / reflux;
|
|
|
With
O,O-Diethyl hydrogen phosphorodithioate; malononitrile;
In
tetrahydrofuran;
Conversion of starting material;
Heating / reflux;
|
|
|
With
hydrogen sulfide; triethylamine;
In
ethanol; toluene;
under 37.5038 Torr;
|
|
|
With
hydrogen sulfide; diethylamine;
In
N,N-dimethyl-formamide;
|
|
|
With
tetraphosphorus decasulfide;
In
ethanol;
at 0 - 60 ℃;
|
|
|
thiophene-2-carbonitrile;
With
sodium hydrogensulfide; 2,2'-[1,2-ethanediylbis(oxy)]bisethanol;
at 20 ℃;
With
sulfuric acid;
at 110 ℃;
for 72h;
|
|
|
With
pyridine; diammonium sulfide; triethylamine;
In
water;
at 50 ℃;
|
-
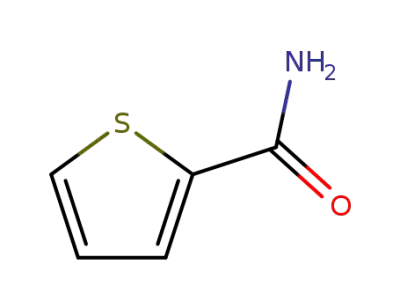
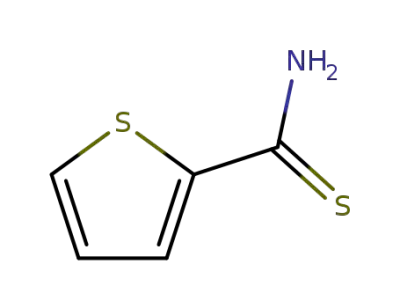
-
5813-89-8
2-thiophenylcarboxamide

-

-
20300-02-1
thiophene-2-carbothioamide
| Conditions | Yield |
|---|---|
|
With
Lawessons reagent;
In
1,2-dimethoxyethane;
at 20 ℃;
for 12h;
Inert atmosphere;
|
88% |
|
With
Lawessons reagent;
In
chlorobenzene;
at 130 ℃;
for 12h;
|
65% |
|
With
Lawessons reagent;
In
chlorobenzene;
at 130 ℃;
for 12h;
|
65% |
|
With
P2S5/alumina;
In
tetrahydrofuran;
at 60 ℃;
for 0.333333h;
Microwave irradiation;
|
63% |
|
With
Lawessons reagent;
In
toluene;
at 95 ℃;
Inert atmosphere;
|
51% |
|
With
Lawessons reagent;
In
1,2-dimethoxyethane;
at 20 ℃;
for 12h;
Inert atmosphere;
|
|
|
With
Lawessons reagent;
In
tetrahydrofuran;
for 4h;
Reflux;
|
20300-02-1 Upstream products
-
1003-31-2

thiophene-2-carbonitrile
-
188290-36-0
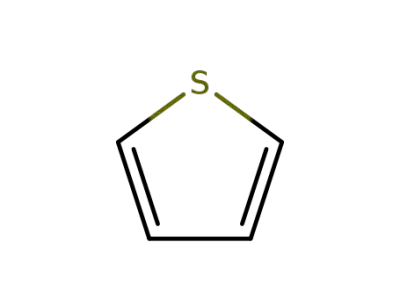
thiophene
-
333-20-0
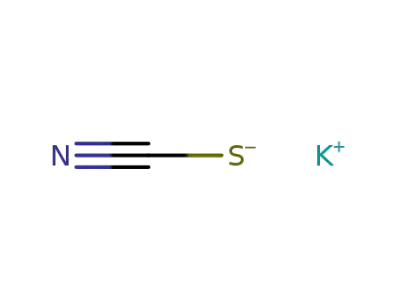
potassium thioacyanate
-
57784-57-3
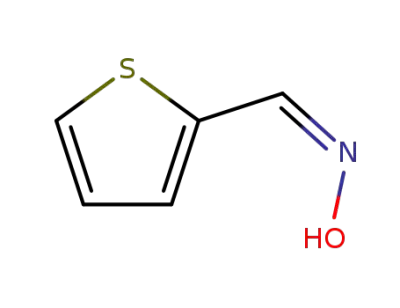
(Z)-thiophene-2-carbaldehyde oxime
20300-02-1 Downstream products
-
54679-16-2
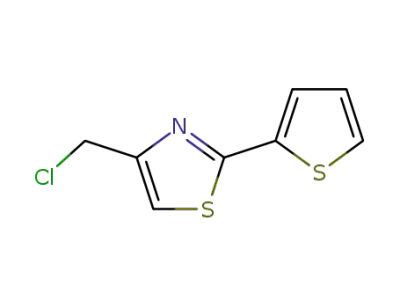
4-(chloromethyl)-2-(thiophen-2-yl)-1,3-thiazole
-
157252-28-3
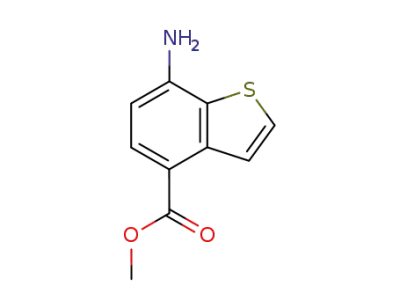
7-Amino-benzo[b]thiophene-4-carboxylic acid methyl ester
-
3319-99-1
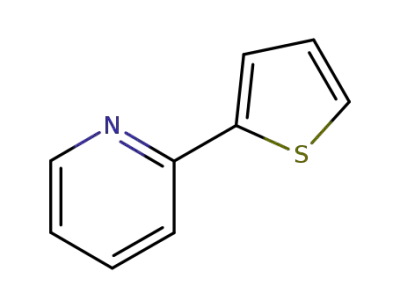
2'-(2-thienyl)pyridine
-
35299-71-9
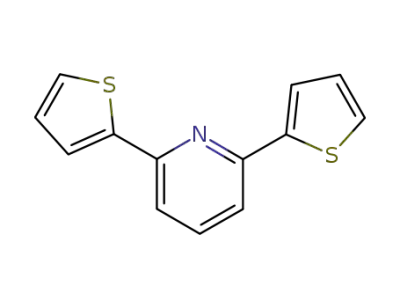
2,6-bis(thiophen-2-yl)pyridine
Relevant Products
-
1-Butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide
CAS:174899-83-3
-
3-Chloro-2-nitrotoluene
CAS:5367-26-0
-
4,4-Biphenyldisulfonic Acid
CAS:5314-37-4