3172-56-3
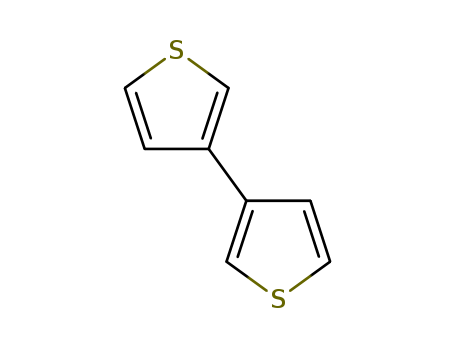
- Product Name:3,3'-Bithiophene
- Molecular Formula:C8H6S2
- Purity:99%
- Molecular Weight:166.268
Product Details;
CasNo: 3172-56-3
Molecular Formula: C8H6S2
Appearance: solid
factory and supplier 3172-56-3 3,3'-Bithiophene in stock
- Molecular Formula:C8H6S2
- Molecular Weight:166.268
- Appearance/Colour:solid
- Melting Point:132-135 °C
- Boiling Point:197.6 °C at 760 mmHg
- Flash Point:50.9 °C
- PSA:56.48000
- Density:1.243 g/cm3
- LogP:3.47660
3172-56-3 Relevant articles
-
Tamaru et al.
, p. 3365 (1977)
-
AN EFFICIENT ROUTE TO HETEROARENE-SUBSTITUTED VINYL- AND ALLYL-SILANES VIA PALLADIUM-PHOSPHINE COMPLEX CATALYZED CROSS-COUPLING
Minato, Akio,Suzuki, Keizo
, p. 83 - 86 (1984)
Heteroarene-substituted vinyl- and allyl...
Synthesis of 6-thienyl-substituted 2-amino-3-cyanopyridines
Verbitskiy,Cheprakova,Pervova,Danagulyan,Rusinov,Chupakhin,Charushin
, p. 689 - 694 (2015)
An efficient method for the synthesis of...
Photoinduced Regioselective Olefination of Arenes at Proximal and Distal Sites
Ali, Wajid,Anjana, S. S.,Bhattacharya, Trisha,Chandrashekar, Hediyala B.,Goswami, Nupur,Guin, Srimanta,Maiti, Debabrata,Panda, Sanjib,Prakash, Gaurav,Saha, Argha,Sasmal, Sheuli,Sinha, Soumya Kumar
supporting information, p. 1929 - 1940 (2022/02/01)
The Fujiwara-Moritani reaction has had a...
Three-dimensional covalent organic frameworks based on a π-conjugated tetrahedral node
Gu, Zhangjie,Shan, Zhen,Wang, Jinjian,Wu, Miaomiao,Wu, Xiaowei,Xu, Bingqing,Zhang, Gen
supporting information, p. 10379 - 10382 (2021/10/12)
The construction of three-dimensional (3...
"benchtop" Biaryl Coupling Using Pd/Cu Cocatalysis: Application to the Synthesis of Conjugated Polymers
Minus, Matthew B.,Moor, Sarah R.,Pary, Fathima F.,Nirmani,Chwatko, Malgorzata,Okeke, Brandon,Singleton, Josh E.,Nelson, Toby L.,Lynd, Nathaniel A.,Anslyn, Eric V.
supporting information, p. 2873 - 2877 (2021/05/05)
Typically, Suzuki couplings used in poly...
Photoelectric properties of aromatic triangular tri-palladium complexes and their catalytic applications in the Suzuki-Miyaura coupling reaction
Li, Jia,Li, Xujun,Liu, Xiang,Maestri, Giovanni,Malacria, Max,Wang, Xiaoshuang,Wang, Yanlan,Wu, Lingang
supporting information, p. 11834 - 11842 (2021/09/06)
The photoelectric properties and catalyt...
3172-56-3 Process route
-
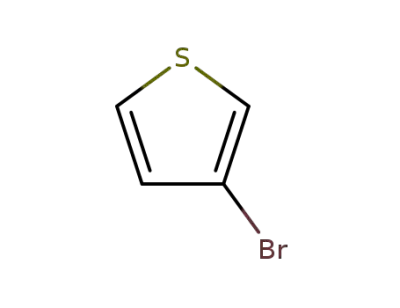
-
872-31-1
3-Bromothiophene

-
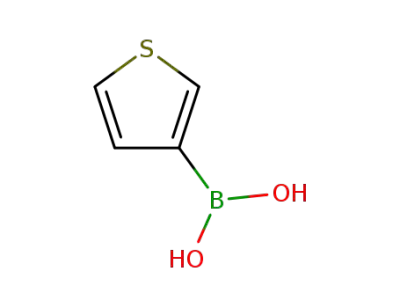
-
6165-69-1
Thien-3-ylboronic acid

-
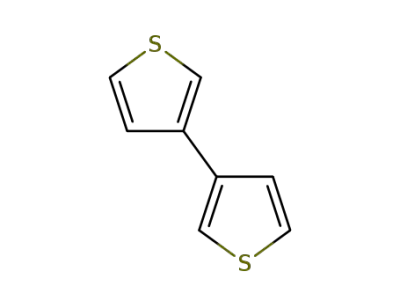
-
3172-56-3
3,3'-bithiophene
| Conditions | Yield |
|---|---|
|
With
bis(dicyclopentyl(2-methoxyphenyl)phosphine)dichloropalladium(II);
In
butan-1-ol;
at 100 ℃;
for 4h;
Cooling;
|
98% |
|
With
tetrakis(triphenylphosphine) palladium(0); sodium carbonate;
In
ethanol; water; toluene;
for 20h;
Inert atmosphere;
Reflux;
|
87% |
|
With
potassium hydroxide;
palladium diacetate; triphenylphosphine;
In
1,2-dimethoxyethane; water;
at 80 ℃;
for 9.25h;
|
76.9% |
|
With
sodium carbonate;
tetrakis(triphenylphosphine) palladium(0);
In
1,2-dimethoxyethane; water;
for 10h;
Heating / reflux;
|
55% |
|
With
tetrakis(triphenylphosphine) palladium(0);
In
tetrahydrofuran; water;
|
-

-
872-31-1
3-Bromothiophene

-

-
3172-56-3
3,3'-bithiophene
| Conditions | Yield |
|---|---|
|
With
(2-hydroxyethyl)ammonium formate; palladium dichloride;
at 100 ℃;
for 2h;
|
96% |
|
With
PEG 4000; palladium diacetate; potassium carbonate;
at 120 ℃;
for 7h;
|
95% |
|
With
triethylamine;
palladium dichloride;
at 80 ℃;
for 5h;
|
94% |
|
With
2Pd(2+)*4Br(1-)*C56H102N4; potassium carbonate;
at 75 ℃;
for 12h;
|
93% |
|
With
[(PhNH)P2(NPh)2]2NPh; triethylamine; palladium dichloride;
In
water;
for 2h;
Reflux;
|
91% |
|
With
palladium diacetate; sodium hydroxide; agarose;
In
water;
at 90 ℃;
for 2h;
|
91% |
|
With
triethylamine; palladium dichloride;
at 20 - 100 ℃;
for 1.45h;
Green chemistry;
|
90% |
|
With
DPEPhos; potassium tert-butylate; palladium diacetate; bis(pinacol)diborane;
for 12h;
Heating;
|
89% |
|
With
[1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene](3-chloropyridyl)palladium(ll) dichloride; tert.-butyl lithium;
In
n-heptane;
at 20 ℃;
for 0.166667h;
Schlenk technique;
|
89% |
|
3-Bromothiophene;
With
n-butyllithium;
In
diethyl ether;
at -78 ℃;
With
copper dichloride;
In
diethyl ether;
Further stages.;
|
87% |
|
With
tetrabutylammonium acetate; palladium diacetate; propionaldehyde;
at 90 ℃;
for 1h;
Inert atmosphere;
|
86% |
|
With
D-glucose; tetra(n-butyl)ammonium hydroxide; palladium diacetate;
In
water;
at 40 ℃;
for 6h;
|
86% |
|
3-Bromothiophene;
With
tert.-butyl lithium;
In
diethyl ether;
at -78 ℃;
In
diethyl ether;
With
Duroquinone;
In
diethyl ether;
|
82% |
|
With
iron(III) trifluoromethanesulfonate; magnesium;
In
tetrahydrofuran;
at 20 ℃;
for 4h;
|
81% |
|
With
manganese; zirconocene dichloride; [(2,9-dimethyl-1,10-phenanthroline)dichloro nickel(II)]; lithium chloride;
In
1,2-dimethoxyethane;
at 20 ℃;
for 48h;
Inert atmosphere;
|
80% |
|
With
potassium phosphate; triphenylphosphine; bis(pinacol)diborane;
cyclopalladated ferrocenylimine;
In
N,N-dimethyl-formamide;
at 100 ℃;
for 14h;
|
78% |
|
3-Bromothiophene;
With
n-butyllithium;
In
diethyl ether; hexane;
at -78 - -60 ℃;
for 1.16667h;
Inert atmosphere;
With
copper dichloride;
In
diethyl ether; hexane;
at -60 - 20 ℃;
for 19h;
Inert atmosphere;
|
77.9% |
|
3-Bromothiophene;
With
n-butyllithium;
In
diethyl ether; hexane;
at -78 - -60 ℃;
for 1.16667h;
Inert atmosphere;
With
copper dichloride;
In
diethyl ether; hexane;
at -60 - 20 ℃;
Inert atmosphere;
|
77.9% |
|
3-Bromothiophene;
With
n-butyllithium;
In
diethyl ether; hexane;
at -78 - -60 ℃;
for 1.16667h;
Inert atmosphere;
With
copper dichloride;
In
diethyl ether; hexane;
at -60 - 20 ℃;
for 19h;
Inert atmosphere;
|
77.9% |
|
3-Bromothiophene;
With
n-butyllithium;
In
diethyl ether; hexane;
at -78 - -60 ℃;
for 1.16667h;
Schlenk technique;
Inert atmosphere;
With
copper dichloride;
In
diethyl ether; hexane;
at -60 - 20 ℃;
for 19h;
Schlenk technique;
Inert atmosphere;
|
76% |
|
With
n-butyllithium; copper dichloride;
In
diethyl ether;
at -78 - 20 ℃;
Inert atmosphere;
|
73.9% |
|
With
N,N,N,N,N,N-hexamethylphosphoric triamide; samarium; nickel dichloride;
In
tetrahydrofuran;
for 24h;
chemoselective reaction;
Inert atmosphere;
Reflux;
|
73% |
|
With
potassium carbonate;
In
ethanol; water;
at 20 ℃;
for 12h;
|
73% |
|
3-Bromothiophene;
With
n-butyllithium;
In
diethyl ether; hexane;
at -70 ℃;
for 0.25h;
With
copper dichloride;
In
diethyl ether; hexane;
at -45 ℃;
for 1h;
|
71% |
|
With
tetrabutylammomium bromide; potassium carbonate; hydroquinone;
dichloro bis(acetonitrile) palladium(II); N,N'-dicyclohexylethylenediimine;
In
N,N-dimethyl-formamide;
at 135 ℃;
for 24h;
|
71% |
|
With
palladium diacetate; bis(tri-n-butyltin); cesium fluoride; tricyclohexylphosphine;
In
neat (no solvent);
at 110 ℃;
for 24h;
|
69% |
|
With
thio-xanthene-9-one; [4,4′-bis(1,1-dimethylethyl)-2,2′-bipyridine]nickel(II) dichloride; N-ethyl-N,N-diisopropylamine;
In
tert-butyl alcohol;
at 20 ℃;
for 12h;
Schlenk technique;
Irradiation;
|
68% |
|
3-Bromothiophene;
With
n-butyllithium;
In
diethyl ether; hexane;
at -70 - -55 ℃;
With
copper dichloride;
In
diethyl ether; hexane;
at -45 ℃;
for 1h;
|
65% |
|
3-Bromothiophene;
With
n-butyllithium;
In
diethyl ether;
at -78 ℃;
Inert atmosphere;
With
copper(l) chloride;
In
diethyl ether;
for 3h;
Inert atmosphere;
Reflux;
|
64% |
|
With
[2,2]bipyridinyl; nickel diacetate; sodium tert-pentoxide;
In
tetrahydrofuran;
at 25 ℃;
for 0.000833333h;
|
60% |
|
3-Bromothiophene;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
With
copper dichloride;
|
60% |
|
With
bis(bipyridine)nickel(II) bromide; ethylene dibromide; sodium iodide;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 5h;
Electrochemical reaction;
Inert atmosphere;
|
48% |
|
With
manganese; tetrakis(triphenylphosphine) palladium(0); ethylene dibromide;
Yield given. Multistep reaction;
1.) THF, 5 h, room t., 2.) THF, 0 deg C to room t., 20 min, 3.) THF, room t., 30 min;
|
|
|
With
pyridine; tetrabutylammonium tetrafluoroborate;
Ethyl 4-bromobenzoate;
In
acetonitrile;
at 20 ℃;
Electrolysis;
iron rod as anode; stainless steel grid as cathode;
|
|
|
3-Bromothiophene;
With
TurboGrignard;
With
synthetic air; cobalt(II) chloride;
|
|
|
3-Bromothiophene;
With
n-butyllithium;
In
diethyl ether;
at -78 ℃;
for 0.25h;
Inert atmosphere;
With
copper dichloride;
In
diethyl ether;
at -55 - 20 ℃;
|
|
|
3-Bromothiophene;
With
n-butyllithium;
In
diethyl ether;
at -78 ℃;
With
copper dichloride;
In
diethyl ether;
at -78 ℃;
|
3172-56-3 Upstream products
-
110-01-0
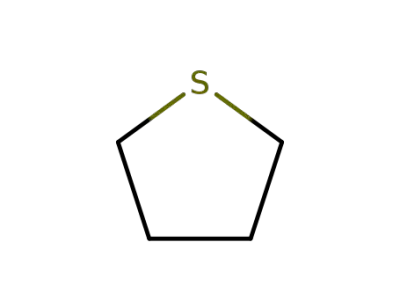
thiophene
-
188290-36-0
thiophene
-
17249-80-8
3-chlorothiophene
-
872-31-1

3-Bromothiophene
3172-56-3 Downstream products
-
18592-86-4
2,2′-dibromo-[3,3′]bithiophene
-
24243-31-0
benzo[1,2-b:4,3-b’]dithiophene-4,5-quinone
-
18592-87-5
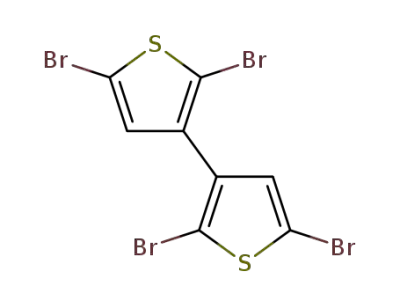
2,2’,5,5’-tetrabromo-3,3’-dithienyl
-
82080-39-5
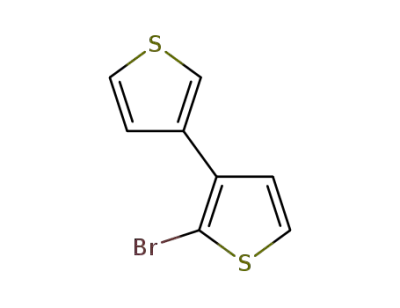
2-bromo-3,3'-dithiophene
Relevant Products
-
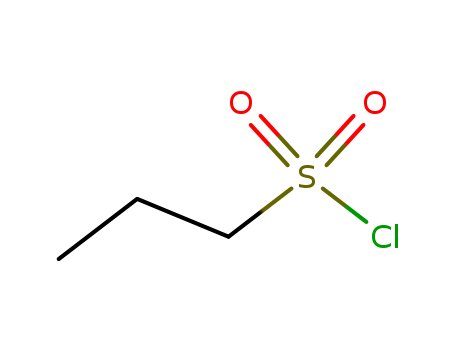
Propanesulphonyl chloride
CAS:10147-36-1
-
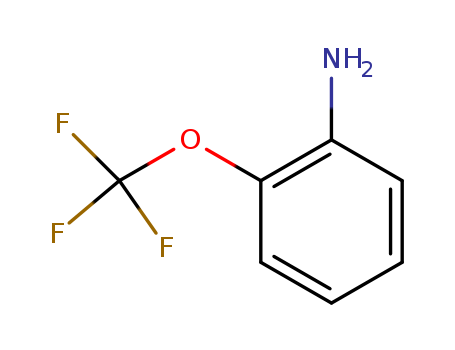
2-(Trifluoromethoxy)aniline
CAS:1535-75-7
-
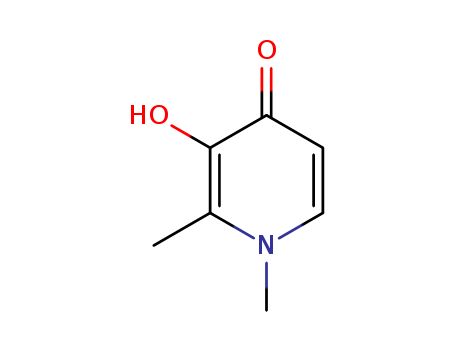
3-Hydroxy-1,2-dimethyl-4(1H)-pyridone
CAS:30652-11-0