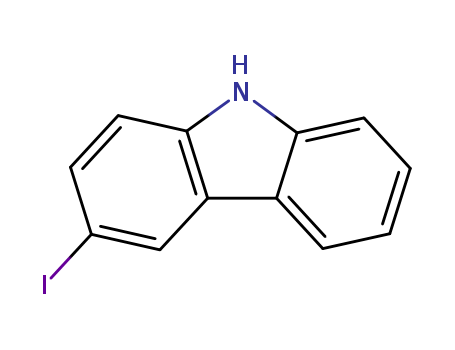
16807-13-9
- Product Name:3-Iodo-9H-carbazole
- Molecular Formula:C12H8IN
- Purity:99%
- Molecular Weight:293.107
Product Details;
CasNo: 16807-13-9
Molecular Formula: C12H8IN
factory and supplier 16807-13-9 3-Iodo-9H-carbazole in stock
- Molecular Formula:C12H8IN
- Molecular Weight:293.107
- Melting Point:195-197℃
- Boiling Point:430.6±18.0 °C(Predicted)
- PKA:16.54±0.30(Predicted)
- Density:1.854
16807-13-9 Relevant articles
A wet- and dry-process feasible carbazole type host for highly efficient phosphorescent OLEDs
Jou, Jwo-Huei,Sahoo, Snehasis,Kumar, Sudhir,Yu, Hui-Huan,Fang, Po-Hsun,Singh, Meenu,Krucaite, Gintare,Volyniuk, Dmytro,Grazulevicius, Juozas Vidas,Grigalevicius, Saulius
, p. 12297 - 12307 (2015)
A wet- and dry-process feasible host mat...
Monomers and oligomers with the pendent adducts of carbazole with 5H-dibenz(b,f)azepine and its 10,11-dihydro derivative
Tomkeviciene,Bartiuk,Bucinskas,Grazulevicius,Jankauskas
, p. 796 - 802 (2011)
The synthesis of a series of carbazole-b...
Synthesis of Novel Derivatives of Carbazole-Thiophene, Their Electronic Properties, and Computational Studies
Damit,Nordin,Ariffin,Sulaiman
, (2016)
A series of carbazole-thiophene dimers, ...
A useful procedure for diiodination of carbazoles and subsequent efficient transformation to novel 3,6-bis(triethoxysilyl)carbazoles giving mesoporous materials
Maegawa, Yoshifumi,Goto, Yasutomo,Inagaki, Shinji,Shimada, Toyoshi
, p. 6957 - 6960 (2006)
Bis(pyridine)iodonium tetrafluoroborate ...
Synthesis and isolation of iodocarbazoles. Direct iodination of carbazoles by N-iodosuccinimide and N-iodosuccinimide-silica gel system
Bonesi,Erra-Balsells
, p. 77 - 87 (2001)
Carbazole (1) undergoes electrophilic ar...
Synthesis, photophysical, electrochemical and electrochemiluminescence properties of A2B2 zinc porphyrins: The effect of π-extended conjugation
Galván-Miranda, Elizabeth K.,Castro-Cruz, Hiram M.,Arturo Arias-Orea,Iurlo, Matteo,Valenti, Giovanni,Marcaccio, Massimo,Macías-Ruvalcaba, Norma A.
, p. 15025 - 15038 (2016)
The synthesis of two A2B2 porphyrins, {5...
A study of the properties, reactivity and anticancer activity of novel N-methylated-3-thiazolyl or 3-thienyl carbazoles and their Pd(II) and Pt(II) complexes
Reig, Marta,Bosque, Ramón,Font-Bardía, Mercè,Calvis, Carme,Messeguer, Ramon,Baldomà, Laura,Badía, Josefa,Velasco, Dolores,López, Concepción
, p. 134 - 145 (2018)
The synthesis and characterization of tw...
Synthesis and properties of bipolar derivatives of 1,3,5-triazine and carbazole
Matulaitis,Kostiv,Grazulevicius,Peciulyte,Simokaitiene,Jankauskas,Luszczynska,Ulanski
, p. 45 - 58 (2016)
Three new bipolar star-shaped derivative...
A one-pot direct iodination of the Fischer-Borsche ring using molecular iodine and its utility in the synthesis of 6-oxygenated carbazole alkaloids
Naykode, Mahavir S.,Humne, Vivek T.,Lokhande, Pradeep D.
, p. 2392 - 2396 (2015)
An efficient regioselective iodination o...
Synthesis and properties of photocross-linkable carbazole dimers
Simkus,Tomkeviciene,Volyniuk,Kostjuk,Grazulevicius
, p. 47 - 54 (2017)
New carbazole-based monomers with two re...
Triazine-based aromatic amines as new glass-forming charge transport materials
Vaitkeviciene,Grazulevicius,Peciuraite,Grigalevicius,Jankauskas
, p. 141 - 150 (2007)
Various diarylamino-substituted 1,3,5-tr...
Study of photochemical cytosine to uracil transition via ultrafast photo-cross-linking using vinylcarbazole derivatives in duplex DNA
Sethi, Siddhant,Nakamura, Shigetaka,Fujimoto, Kenzo
, (2018)
Gene therapies, including genome editing...
Enantioselective recognition of tartaric acids with ethynylated carbazole-based chiral bisboronic acid chemosensors with improved response at acidic pH
Liu, Yifan,Zhang, Xin,Guo, Huimin,Wu, Yubo,Li, Qiuting,Liu, Liping,Zhao, Jianzhang
, p. 1979 - 1986 (2011)
Chiral bisboronic acid chemosensors base...
Generation of electrophilic iodine from iodine monochloride in neutral media. Iodination and protodeiodination of carbazole
Filimonov,Krasnokutskaya,Lesina
, p. 875 - 880 (2003)
In reaction of iodine monochloride with ...
Solution-processable naphthalene and phenyl substituted carbazole core based hole transporting materials for efficient organic light-emitting diodes
Kumar, Sudhir,An, Chih-Chia,Sahoo, Snehasis,Griniene, Raimonda,Volyniuk, Dmytro,Grazulevicius, Juozas V.,Grigalevicius, Saulius,Jou, Jwo-Huei
, p. 9854 - 9864 (2017)
Solution-processable molecular hole tran...
Oligomers containing pyridinyl-substituted carbazole rings as host materials for phosphorescent OLEDs
Krucaite,Tavgeniene,Blazevicius,Baranauskyte,Zaleckas
, p. 160 - 167 (2018)
Two oligoethers containing electroactive...
Easy accessible blue luminescent carbazole-based materials for organic light-emitting diodes
Reig, Marta,Gozálvez, Cristian,Bujaldón, Roger,Bagdziunas, Gintautas,Ivaniuk, Khrystyna,Kostiv, Nataliya,Volyniuk, Dmytro,Grazulevicius, Juozas V.,Velasco, Dolores
, p. 24 - 35 (2017)
The thermal, optical, electrochemical an...
(Bi)phenyl substituted 9-(2,2-diphenylvinyl)carbazoles as low cost hole transporting materials for efficient red PhOLEDs
Grigalevicius, Saulius,Tavgeniene, Daiva,Krucaite, Gintare,Griniene, Raimonda,Wang, Yen-Po,Tsai, Shang-Ru,Chang, Chih-Hao
, p. 173 - 178 (2018)
Two low-cost 9-(2,2-diphenylvinyl)carbaz...
Dynamic Generation of G-Quadruplex DNA Ligands by Target-Guided Combinatorial Chemistry on a Magnetic Nanoplatform
Jana, Snehasish,Panda, Deepanjan,Saha, Puja,Pantos, G. Dan,Dash, Jyotirmayee
, p. 762 - 773 (2019)
Dynamic combinatorial chemistry (DCC) ha...
Synthesis and properties of 1,3,5-tricarbazolylbenzenes with star-shaped architecture
Brzeczek, Alina,Ledwon, Przemyslaw,Data, Przemyslaw,Zassowski, Pawel,Golba, Sylwia,Walczak, Krzysztof,Lapkowski, Mieczyslaw
, p. 640 - 648 (2015)
A series of star-shaped carbazole deriva...
Synthesis and Properties of Blue Fluorescent Dyes Based on Diphenylamine by Introducing an Acetylene Linkage Group with Carbazole and 1,8-Naphthalimide
Kim, Tae-Heon,Kwon, Su-Hyeon,Choi, Jae-Hong
, p. 1229 - 1232 (2019)
-
Efficient Hydro- and Organogelation by Minimalistic Diketopiperazines Containing a Highly Insoluble Aggregation-Induced, Blue-Shifted Emission Luminophore**
Molkenthin, Martin,Nachtsheim, Boris J.,Nau, Werner M.
supporting information, p. 16488 - 16497 (2021/10/25)
We report the synthesis, gelation abilit...
Carbazole modified oligonucleotides: Synthesis, hybridization studies and fluorescence properties
Gouda, Alaa S.,J?rgensen, Per T.,Przypis, ?ukasz,Walczak, Krzysztof,Wengel, Jesper
, p. 6935 - 6948 (2020/10/02)
Synthesis of the novel thiophenyl carbaz...
Synthesis and photophysical properties of fluorescent dyes based on triphenylamine, diphenylamine, diphenyl sulfone or triphenyltriazine derivatives containing an acetylene linkage group
Ahn, Sung-Ok,Choi, Jae-Hong,Kim, Kyung-Won,Kwon, Su-Hyeon,Lee, Byung-Jun,Lee, Ju-Hong
, (2020/06/22)
In this study, ten fluorescent dyes were...
Long-Lived Triplet Excited State Accessed with Spin–Orbit Charge Transfer Intersystem Crossing in Red Light-Absorbing Phenoxazine-Styryl BODIPY Electron Donor/Acceptor Dyads
Dick, Bernhard,Dong, Yu,Elmali, Ayhan,Karatay, Ahmet,Zhao, Jianzhang
, (2020/06/08)
Orthogonal phenoxazine-styryl BODIPY com...
16807-13-9 Process route
-
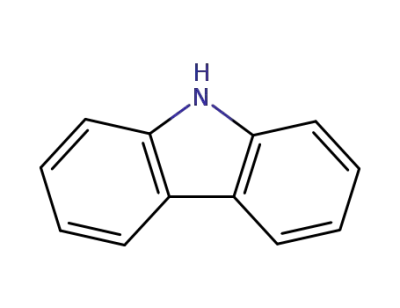
-
86-74-8,105184-46-1,97960-57-1
9H-carbazole

-
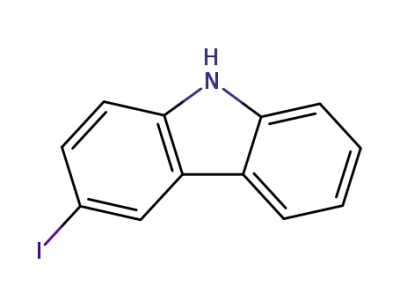
-
16807-13-9
3-iodocarbazole
| Conditions | Yield |
|---|---|
|
With
N-iodo-succinimide;
In
acetic acid;
at 20 ℃;
|
97% |
|
9H-carbazole;
With
N-iodo-succinimide; acetic acid;
at 20 ℃;
for 12h;
With
sodium hydrogencarbonate;
In
water; ethyl acetate;
|
97% |
|
With
iodine;
In
dimethyl sulfoxide;
at 110 ℃;
for 6h;
Temperature;
Concentration;
regioselective reaction;
|
95% |
|
With
N-iodo-succinimide;
In
acetic acid;
at 20 ℃;
for 1h;
|
86% |
|
With
diphenyliodonium tetrafluoroborate; copper(II) sulfate;
In
acetonitrile;
at 65 ℃;
for 0.166667h;
Inert atmosphere;
Schlenk technique;
|
79% |
|
With
[bis(pyridine)iodine]+ tetrafluoroborate; copper(II) sulfate;
In
acetonitrile;
at 65 ℃;
for 0.166667h;
Inert atmosphere;
|
79% |
|
With
potassium iodate; potassium carbonate; acetic acid; potassium iodide;
at 80 ℃;
for 5h;
|
72% |
|
9H-carbazole;
With
acetic acid; potassium iodide;
at 110 ℃;
With
potassium iodate;
at 100 ℃;
for 0.166667h;
|
72.7% |
|
With
iodine; silver nitrate; acetic acid;
at 20 ℃;
|
70% |
|
9H-carbazole;
With
potassium iodide;
In
acetic acid;
at 100 ℃;
for 1h;
With
potassium iodate;
In
acetic acid;
at 100 ℃;
for 2h;
|
70% |
|
With
iron(III) chloride; sodium iodide;
In
acetonitrile;
for 8h;
|
68% |
|
9H-carbazole;
With
acetic acid; potassium iodide;
at 85 ℃;
Reflux;
With
potassium iodate;
at 85 ℃;
for 0.166667h;
|
62% |
|
With
sodium periodate; sulfuric acid; iodine;
In
ethanol;
at 60 ℃;
for 2h;
|
49% |
|
With
sodium periodate; sulfuric acid; iodine;
In
isopropyl alcohol;
at 65 ℃;
for 6h;
|
48% |
|
With
potassium iodate; potassium iodide;
In
acetic acid;
at 45 ℃;
|
47% |
|
9H-carbazole;
With
ammonium iodide;
In
methanol;
for 0.0333333h;
In
methanol;
at 75 ℃;
|
47% |
|
With
potassium iodate; potassium iodide;
In
acetic acid;
for 0.166667h;
Reflux;
|
45% |
|
With
potassium iodate; acetic acid; potassium iodide;
for 0.166667h;
Reflux;
|
45% |
|
With
potassium iodate; acetic acid; potassium iodide;
Heating;
|
44.3% |
|
With
N-iodo-succinimide; acetic acid;
at 20 ℃;
for 5h;
Inert atmosphere;
|
44% |
|
With
N-iodo-succinimide; acetic acid;
at 20 ℃;
for 5h;
Inert atmosphere;
|
44% |
|
With
N-iodo-succinimide; acetic acid;
at 20 ℃;
for 5h;
Inert atmosphere;
|
44% |
|
With
N-iodo-succinimide; acetic acid;
at 20 ℃;
for 5h;
Inert atmosphere;
|
44% |
|
With
N-iodo-succinimide; acetic acid;
at 20 ℃;
for 5h;
Inert atmosphere;
|
44% |
|
With
N-iodo-succinimide; acetic acid;
at 20 ℃;
for 5h;
Inert atmosphere;
|
44% |
|
With
potassium iodate; acetic acid; potassium iodide;
at 100 ℃;
for 2h;
|
40% |
|
With
potassium iodate; acetic acid; potassium iodide;
at 85 ℃;
for 0.166667h;
|
39% |
|
With
periodic acid dihydrate; sulfuric acid; iodine;
In
ethanol;
at 20 ℃;
for 4h;
|
18.8% |
|
With
potassium iodate; acetic acid; potassium iodide;
at 80 ℃;
for 1h;
|
15% |
|
With
potassium iodate; potassium iodide;
|
|
|
With
sulfuric acid; iodine; periodic acid;
acetic acid;
In
water;
at 70 ℃;
for 1h;
|
|
|
With
potassium iodate; potassium iodide;
|
|
|
With
potassium iodate; potassium iodide;
In
acetic acid;
|
|
|
With
sulfuric acid; iodine; periodic acid;
In
water; acetic acid;
at 70 ℃;
for 1h;
|
|
|
With
potassium iodate; potassium iodide;
In
acetic acid;
at 50 ℃;
for 2h;
|
|
|
With
potassium iodate; acetic acid; potassium iodide;
|
|
|
With
Iodine monochloride;
In
acetonitrile;
at 0 - 20 ℃;
for 4h;
Inert atmosphere;
|
|
|
With
potassium iodate; acetic acid; potassium iodide;
|
|
|
With
potassium iodate; potassium iodide;
|
|
|
With
Iodine monochloride;
at 0 - 20 ℃;
for 4h;
|
|
|
With
potassium iodate; potassium iodide;
|
|
|
With
Iodine monochloride;
In
acetonitrile;
at 0 - 20 ℃;
for 4h;
Inert atmosphere;
|
|
|
With
potassium iodate; acetic acid; potassium iodide;
Reflux;
|
|
|
With
periodic acid dihydrate; sulfuric acid; iodine;
In
ethanol; water;
for 4h;
|
5.1 g |
|
With
potassium iodate; potassium iodide;
In
acetic acid;
for 0.333333h;
Reflux;
|
|
|
With
potassium iodate; potassium iodide;
|
|
|
With
potassium iodate; acetic acid; potassium iodide;
at 120 ℃;
for 0.166667h;
|
|
|
With
potassium iodate; potassium iodide;
at 130 ℃;
|
|
|
With
sodium periodate; sulfuric acid; iodine;
In
ethanol;
|
|
|
With
sodium periodate; sulfuric acid; iodine;
In
ethanol;
|
|
|
With
potassium iodate; acetic acid; potassium iodide;
Reflux;
|
|
|
With
potassium iodate; acetic acid; potassium iodide;
at 110 ℃;
for 2h;
|
-
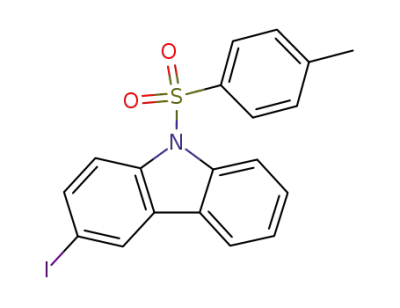
-
861312-65-4
3-iodo-9-(p-tolylsulfonyl)carbazole

-

-
16807-13-9
3-iodocarbazole
| Conditions | Yield |
|---|---|
|
With
sodium hydride;
In
N,N-dimethyl acetamide;
at 60 ℃;
for 3.5h;
Inert atmosphere;
|
85% |
16807-13-9 Upstream products
-
86-74-8

9H-carbazole
-
64-19-7
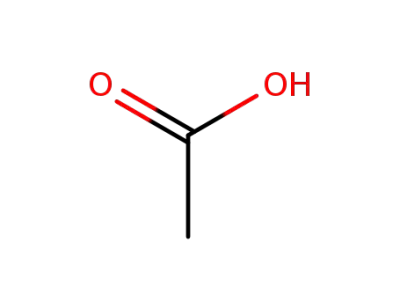
acetic acid
-
942-01-8
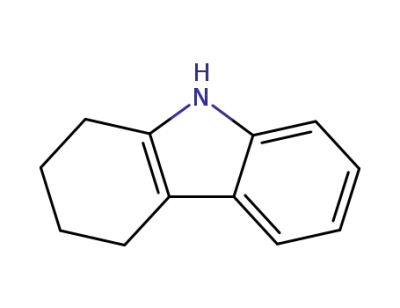
1,2,3,4-tetrahydrocarbazole
-
108-94-1
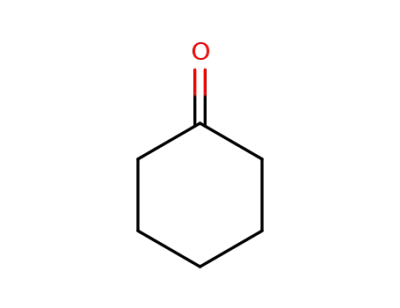
cyclohexanone
16807-13-9 Downstream products
-
861312-65-4

3-iodo-9-(p-tolylsulfonyl)carbazole
-
148291-45-6
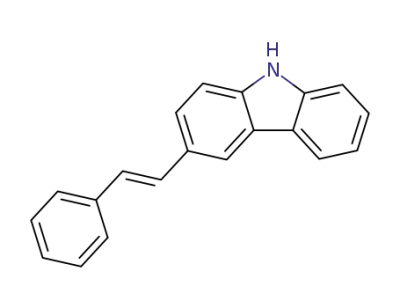
(E)-3-(2-phenylethenyl)carbazole
-
883224-27-9
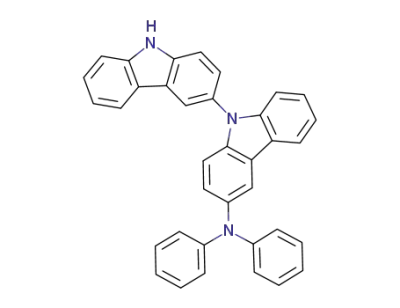
C36H25N3
-
50668-21-8
3-iodo-9-ethylcarbazole
Relevant Products
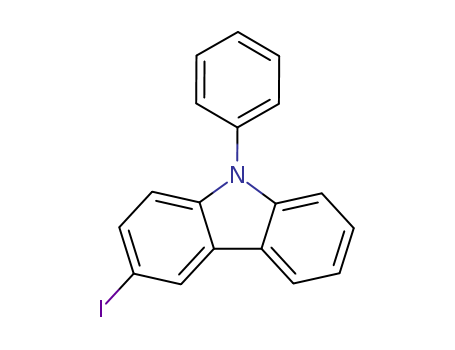
-
3-Iodo-N-phenylcarbazole
CAS:502161-03-7
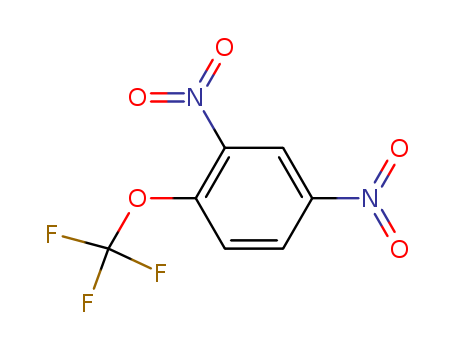
-
2,4-Dinitro-1-(trifluoromethoxy)benzene
CAS:655-07-2
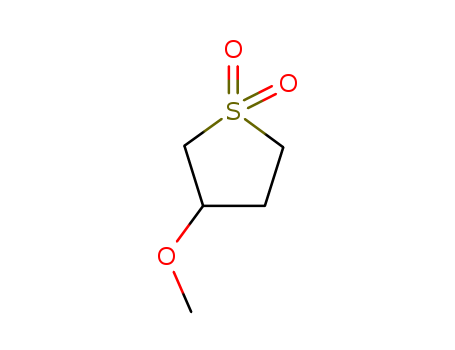
-
3-Methoxysulfolane
CAS:20627-66-1