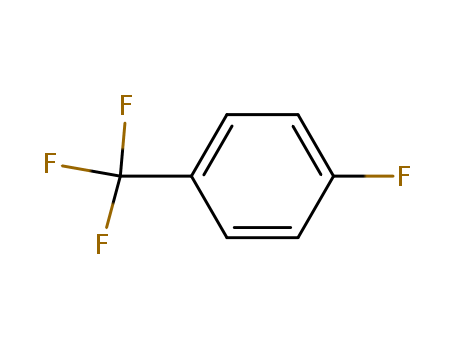
402-44-8
- Product Name:4-Fluorobenzotrifluoride
- Molecular Formula:C7H4F4
- Purity:99%
- Molecular Weight:164.102
Product Details;
CasNo: 402-44-8
Molecular Formula: C7H4F4
Appearance: Clear colourless to light yellow liquid
factory and supplier 402-44-8 4-Fluorobenzotrifluoride in stock
- Molecular Formula:C7H4F4
- Molecular Weight:164.102
- Appearance/Colour:Clear colourless to light yellow liquid
- Vapor Pressure:35.8mmHg at 25°C
- Melting Point:-42--41.7 °C(lit.)
- Refractive Index:n20/D 1.401(lit.)
- Boiling Point:104.5 °C at 760 mmHg
- Flash Point:10.6 °C
- PSA:0.00000
- Density:1.29 g/cm3
- LogP:2.84450
4-Fluorobenzotrifluoride(Cas 402-44-8) Usage
|
Synthesis Reference(s) |
Tetrahedron, 49, p. 8129, 1993 DOI: 10.1016/S0040-4020(01)88032-7 |
InChI:InChI=1/C7H4F4/c8-6-3-1-5(2-4-6)7(9,10)11/h1-4H
402-44-8 Relevant articles
Cross-Coupling through Ag(I)/Ag(III) Redox Manifold
Demonti, Luca,Mézailles, Nicolas,Nebra, Noel,Saffon-Merceron, Nathalie
supporting information, p. 15396 - 15405 (2021/10/12)
In ample variety of transformations, the...
Fluorination of arylboronic esters enabled by bismuth redox catalysis
Planas, Oriol,Wang, Feng,Leutzsch, Markus,Cornella, Josep
, p. 313 - 317 (2020/01/28)
Bismuth catalysis has traditionally reli...
Oxidatively Induced Aryl-CF3 Coupling at Diphosphine Nickel Complexes
Bour, James R.,Roy, Pronay,Canty, Allan J.,Kampf, Jeff W.,Sanford, Melanie S.
supporting information, p. 3 - 7 (2020/01/03)
This communication describes the synthes...
Mechanism of Photoredox-Initiated C-C and C-N Bond Formation by Arylation of IPrAu(I)-CF3 and IPrAu(I)-Succinimide
Kim, Suhong,Toste, F. Dean
, p. 4308 - 4315 (2019/01/25)
Herein, we report on the photoredox-init...
402-44-8 Process route
-
-
C41H32F4FeNiP2

-

-
lithium chloride

-
![[1,1'-bis(diphenylphosphino)ferrocene]nickel(II) chloride](/upload/2026/1/9fb20343-7640-42c5-9d1a-08964b6f50d1.png)
-
67292-34-6
[1,1'-bis(diphenylphosphino)ferrocene]nickel(II) chloride

-
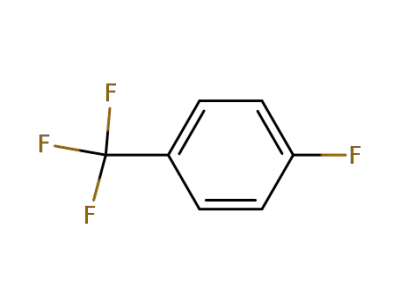
-
402-44-8
4-Fluorobenzotrifluoride
| Conditions | Yield |
|---|---|
|
With
ferrocenium hexafluorophosphate;
In
acetone;
at 23 ℃;
for 0.5h;
Inert atmosphere;
Glovebox;
|
72 %Spectr. |
-
-
C33H28F4NiP2

-
-
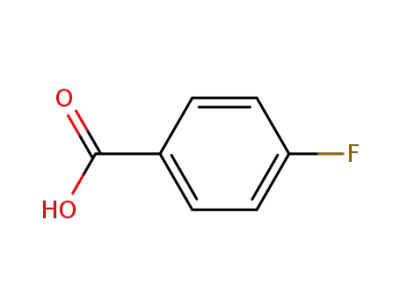
456-22-4
4-Fluorobenzoic acid

-
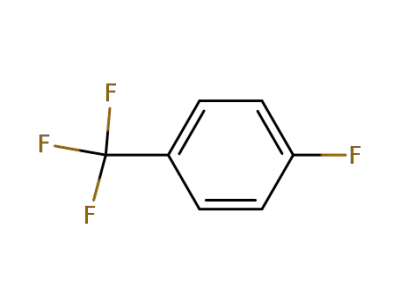
-
402-44-8
4-Fluorobenzotrifluoride
| Conditions | Yield |
|---|---|
|
With
ferrocenium hexafluorophosphate; water;
In
acetone;
at 25 ℃;
for 0.5h;
Inert atmosphere;
Glovebox;
|
65 %Spectr. 18 %Spectr. |
402-44-8 Upstream products
-
402-42-6
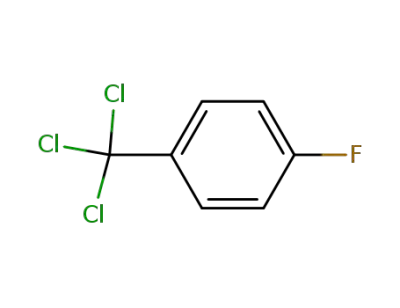
4-fluoro-1-trichloromethylbenzene
-
56-23-5
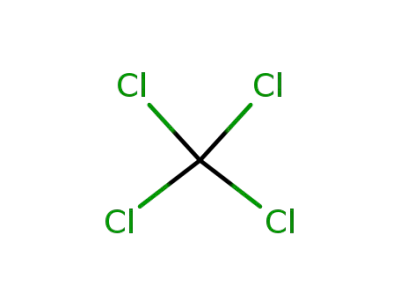
tetrachloromethane
-
462-06-6
fluorobenzene
-
455-14-1
4-trifluoromethylphenylamine
402-44-8 Downstream products
-
367-86-2
1-fluoro-2-nitro-4-trifluoromethyl-benzene
-
115029-23-7
2-fluoro-5-(trifluoromethyl)benzoic acid
-
125923-59-3
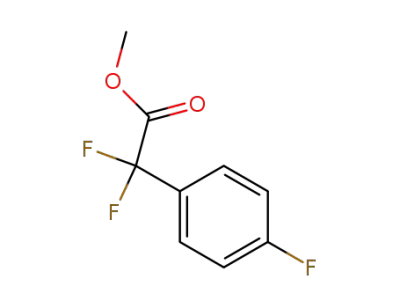
methyl difluoro(4-fluorophenyl)acetate
-
128470-60-0
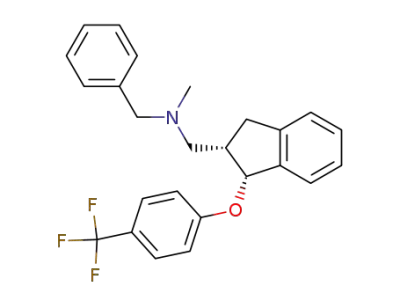
Benzyl-methyl-[1-(4-trifluoromethyl-phenoxy)-indan-2-ylmethyl]-amine
Relevant Products
-
5-Bromo-2-fluoro-m-xylene
CAS:99725-44-7
-
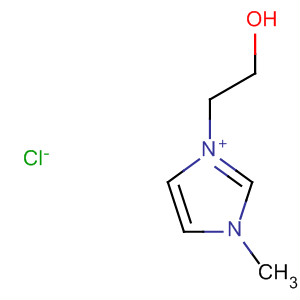
1-(2’-hydroxylethyl)-3-methylimidazolium chloride
CAS:61755-34-8
-
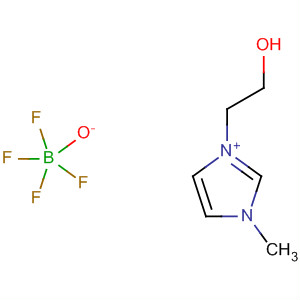
1-(2-Hydroxyethyl)-3-Methylimidazolium Tetrafluoroborate
CAS:374564-83-7