61755-34-8
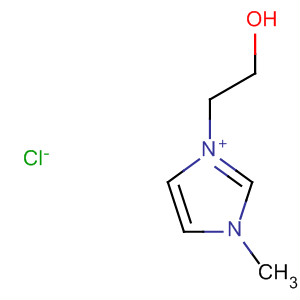
- Product Name:1-(2’-hydroxylethyl)-3-methylimidazolium chloride
- Molecular Formula:C6H11N2O.Cl
- Purity:99%
- Molecular Weight:164.63300
Product Details;
CasNo: 61755-34-8
Molecular Formula: C6H11N2O.Cl
factory and manufacture 61755-34-8 1-(2’-hydroxylethyl)-3-methylimidazolium chloride lonic liquid
- Molecular Formula:C6H11N2O.Cl
- Molecular Weight:164.63300
- Melting Point:86℃
- PSA:26.71000
- LogP:0.33260
61755-34-8 Relevant articles
Degradation of polycarbonate to produce bisphenol A catalyzed by imidazolium-based DESs under metal-and solvent-free conditions
Huang, Wenwen,Wang, Hui,Hu, Weiyue,Yang, Daoshan,Yu, Shitao,Liu, Fusheng,Song, Xiuyan
, p. 1595 - 1604 (2021)
Bisphenol A (BPA) is an important chemic...
Synthesis, structure and properties of imidazolium-based energetic ionic liquids
Yang, Haijun,Liu, Yuejia,Ning, Hongli,Lei, Jianlei,Hu, Gang
, p. 33231 - 33240 (2017)
A series of imidazolium energetic ionic ...
Thermochromism, stability and thermodynamics of cobalt(ii) complexes in newly synthesized nitrate based ionic liquid and its photostability
Bani, Nemanja,Vrane, Milan,Abramovi, Biljana,Csandi, Jnos,Gaduri, Slobodan
, p. 15515 - 15525 (2014)
In this work a 1-(2-hydroxyethyl)-3-meth...
Solubility and diffusion of H2S and CO2 in the ionic liquid 1-(2-Hydroxyethyl)-3-methylimidazolium tetrafluoroborate
Shokouhi, Mohammad,Adibi, Mina,Jalili, Amir Hossein,Hosseini-Jenab, Masih,Mehdizadeh, Ali
, p. 1663 - 1668 (2010)
The solubilities and diffusion coefficie...
Cluster Formation through Hydrogen Bond Bridges across Chloride Anions in a Hydroxyl-Functionalized Ionic Liquid
Panja, Sumit Kumar,Haddad, Boumediene,Debdab, Mansour,Kiefer, Johannes,Chaker, Yassine,Bresson, Serge,Paolone, Annalisa
, p. 936 - 940 (2019)
Several recent studies of hydroxyl-funct...
Hydroxyl-functionalized ionic liquid: a novel efficient catalyst for chemical fixation of CO2 to cyclic carbonate
Sun, Jian,Zhang, Suojiang,Cheng, Weiguo,Ren, Junyi
, p. 3588 - 3591 (2008)
A series of hydroxyl-functionalized ioni...
Nickel and palladium complexes of enolatefunctionalised N-heterocyclic carbenes
Shanmuganathan, Saravanakumar,Kuehl, Olaf,Jones, Peter G.,Heinicke, Joachim
, p. 992 - 998 (2010)
The reaction of chloroethyltrimethylsily...
Preparation and characterization of new room temperature ionic liquids
Branco, Luis C.,Rosa, Joao N.,Moura Ramos, Joaquim J.,Afonso, Carlos A. M.
, p. 3671 - 3677 (2002)
A new series [CnOmmim][X] of imidazolium...
A new approach to N-3 functionalized 3,4-dihydropyrimidine-2(1H)-ones with 1,2,4-oxadiazole group as amide isostere via ionic liquid-phase technology
Legeay, Jean Christophe,Vanden Eynde, Jean Jacques,Bazureau, Jean Pierre
, p. 1063 - 1068 (2007)
New N-3 functionalized 3,4-dihydropyrimi...
Synthesis and characterization of 1-(hydroxyethyl)-3-methylimidazolium sulfate and chloride ionic liquids
Chaker, Yassine,Ilikti, Hocine,Debdab, Mansour,Moumene, Taqiyeddine,Belarbi, El Habib,Wadouachi, Anne,Abbas, Ouissam,Khelifa, Brahim,Bresson, Serge
, p. 182 - 190 (2016)
We have used the imidazole as a starting...
A design of experiment approach for ionic liquid-based extraction of toxic components-minimized essential oil from Myristica fragrans houtt
Lanari, Daniela,Marcotullio, Maria Carla,Neri, Andrea
, (2018)
The effect of the addition of ionic liqu...
Vapor pressures of the 1-butyl-3-methylimidazolium bromide + water, 1-butyl-3-methylimidazolium tetrafluoroborate + water, and 1-(2-hydroxyethyl)-3- methylimidazolium tetrafluoroborate + water systems
Kim, Ki-Sub,Park, Seung-Yeob,Choi, Sukjeong,Lee, Huen
, p. 1550 - 1553 (2004)
This work presents the vapor pressures o...
Synthesis, vibrational and thermal properties of new functionalized 1- (2-hydroxyethyl) -3-methylimidazolium dihydrogenophosphate ionic liquid
Zaoui, Tayeb,Debdab, Mansour,Haddad, Boumediene,Belarbi, El Habib,Chaker, Yassine,Rahmouni, Mustapha,Bresson, Serge,Baeten, Vincent
, (2021)
Very recently, the hydroxyl-functionaliz...
Ionic-liquid-supported total synthesis of sansalvamide A peptide
Chen, Ling,Zheng, Mingfang,Zhou, Yu,Liu, Hong,Jiang, Hualiang
, p. 239 - 248 (2008)
A facile and efficient total synthesis o...
Ionic liquid supported synthesis of β-lactam library in ionic liquid batch
Tao, Xiao-Le,Lei, Ming,Wang, Yan-Guang
, p. 5143 - 5146 (2007)
An efficient and general ionic liquid su...
Merging structural frameworks of imidazolium, pyridinium, and cholinium ionic liquids with cinnamic acid to tune solution state behavior and properties
Ananikov, Valentine P.,Egorova, Ksenia S.,Gordeev, Evgeniy G.,Posvyatenko, Alexandra V.,Seitkalieva, Marina M.,Strukova, Elena N.,Vavina, Anna V.
, (2022/03/14)
Solubility in water, interactions with t...
Design, synthesis and characterization of new energetic phthalate plasticizers based on imidazolium ionic liquids
Fareghi-Alamdari, Reza,Mousavi Nodoushan, Seyed Amanollah,Zekri, Negar
, (2021/08/16)
In this study, a new class of energetic ...
Synthesis and characterization of physicochemical properties of imidazolium-based ionic liquids and their application for simultaneous determination of sulfur compounds
Shoja, Seyed Mohammad Reza,Abdouss, Majid,Beigi, Ali Akbar Miran
, (2021/02/03)
Three types of imidazolium-based ionic l...
61755-34-8 Process route
-
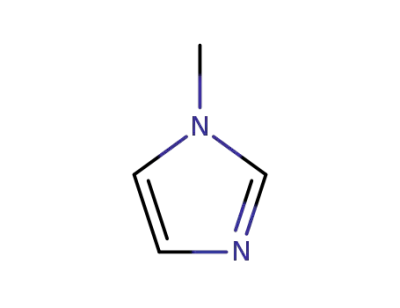
-
616-47-7
1-methyl-1H-imidazole

-
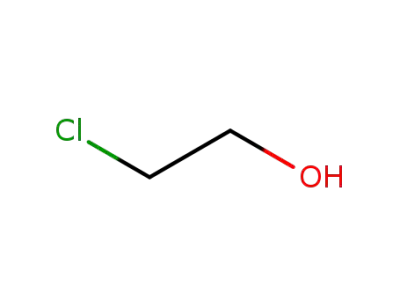
-
107-07-3
2-chloro-ethanol

-
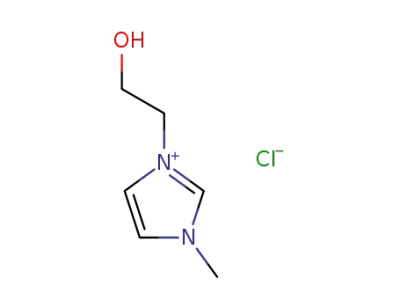
-
61755-34-8
3-(2-hydroxyethyl)-1-methyl-1H-imidazol-3-ium chloride
| Conditions | Yield |
|---|---|
|
at 180 ℃;
for 0.166667h;
microwave irradiation;
|
99% |
|
at 80 ℃;
for 96h;
|
98% |
|
for 0.0333333h;
Heating;
|
96% |
|
at 180 ℃;
for 0.166667h;
microwave irradiation;
|
96% |
|
In
chloroform;
for 8h;
Reflux;
|
95% |
|
at 180 ℃;
for 0.166667h;
microwave irradiation;
|
94% |
|
at 120 ℃;
for 24h;
|
94% |
|
at 120 ℃;
for 24h;
|
94% |
|
at 120 ℃;
for 24h;
|
94.5% |
|
In
neat (no solvent);
at 80 ℃;
for 2h;
|
94% |
|
at 70 ℃;
for 24h;
|
92% |
|
at 79.84 ℃;
for 24h;
|
92.5% |
|
In
toluene;
at 70 ℃;
for 24h;
|
91% |
|
at 60 ℃;
for 24h;
|
90% |
|
at 100 ℃;
for 0.25h;
Microwave irradiation;
|
90% |
|
In
ethanol;
at 80 ℃;
for 20h;
|
88% |
|
at 80 ℃;
for 24h;
|
82% |
|
at 79.84 ℃;
for 24h;
|
80.7% |
|
at 180 ℃;
for 0.0833333h;
Microwave irradiation;
Green chemistry;
|
80% |
|
for 0.5h;
under 7500.75 Torr;
Microwave irradiation;
Heating;
|
66.6% |
|
at 99.84 ℃;
for 4h;
Inert atmosphere;
|
65% |
|
In
acetonitrile;
for 72h;
Reflux;
|
62% |
|
at 80 ℃;
for 120h;
|
|
|
In
acetonitrile;
at 80 ℃;
for 96h;
|
|
|
at 20 ℃;
for 24h;
|
|
|
at 180 ℃;
for 0.166667h;
Microwave irradiation;
|
|
|
In
chloroform;
for 8h;
Reflux;
|
|
|
at 80 ℃;
for 60h;
|
|
|
at 80 ℃;
for 96h;
Schlenk technique;
|
|
|
In
ethyl acetate;
at 78.84 ℃;
for 168h;
Reflux;
|
|
|
In
acetonitrile;
for 48h;
Reflux;
|
|
|
at 79.97 ℃;
for 24h;
|
|
|
at 80 ℃;
Schlenk technique;
|
|
|
In
acetonitrile;
for 28h;
Reflux;
|
|
|
at 80 ℃;
for 12h;
|
|
|
at 50 ℃;
for 12h;
|
|
|
at 80 ℃;
for 20h;
|
|
|
In
toluene;
at 80 ℃;
for 24h;
|
|
|
at 80 ℃;
Schlenk technique;
|
|
|
for 1h;
Reflux;
|
|
|
In
acetonitrile;
at 119.99 ℃;
for 24h;
Inert atmosphere;
|
|
|
at 120 ℃;
for 24h;
|
|
|
at 100 ℃;
for 84h;
Inert atmosphere;
|
-

-
107-07-3
2-chloro-ethanol

-

-
61755-34-8
3-(2-hydroxyethyl)-1-methyl-1H-imidazol-3-ium chloride
| Conditions | Yield |
|---|---|
|
With
1-methyl-1H-imidazole;
In
toluene;
|
61755-34-8 Upstream products
-
616-47-7

1-methyl-1H-imidazole
-
107-07-3

2-chloro-ethanol
-
96-24-2
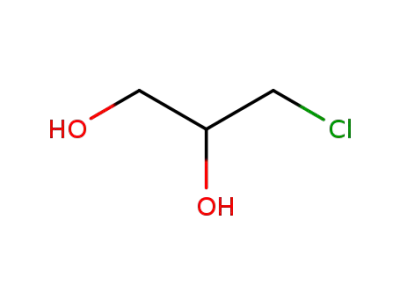
3-monochloro-1,2-propanediol
-
1261121-13-4
3-methyl-1-(2-trimethylsilyloxy)ethylimidazolium chloride
Relevant Products
-
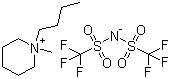
1-Butyl-1-methylpiperidinium bis(trifluoromethylsulfonyl)imide
CAS:623580-02-9
-
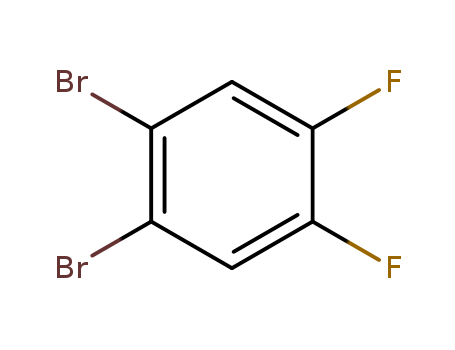
1,2-Dibromo-4,5-difluorobenzene
CAS:64695-78-9
-
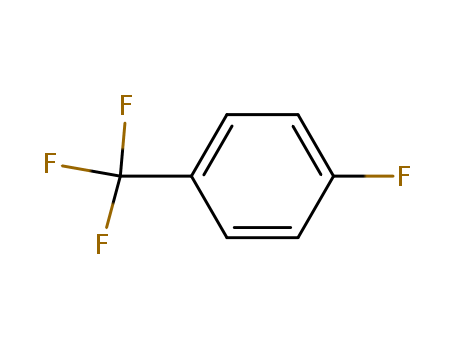
4-Fluorobenzotrifluoride
CAS:402-44-8