31486-86-9
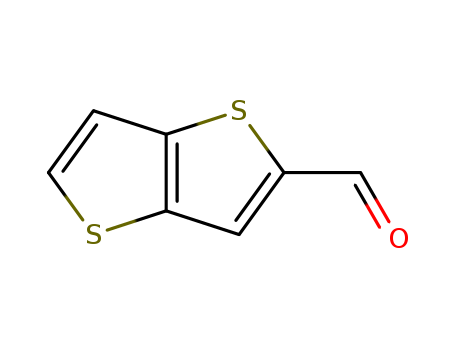
- Product Name:2-Formylthieno[3,2-b]thiophene
- Molecular Formula:C7H4 O S2
- Purity:99%
- Molecular Weight:168.24
Product Details;
CasNo: 31486-86-9
Molecular Formula: C7H4 O S2
factory and supplier 31486-86-9 2-Formylthieno[3,2-b]thiophene in stock
- Molecular Formula:C7H4 O S2
- Molecular Weight:168.24
- Vapor Pressure:0.000679mmHg at 25°C
- Melting Point:53-54℃
- Refractive Index:1.773
- Boiling Point:308.5°Cat760mmHg
- Flash Point:140.4°C
- PSA:73.55000
- Density:1.473g/cm3
- LogP:2.77530
THIENO[3,2-B!THIOPHENE-2-CARBALDEHYDE, 97+%(Cas 31486-86-9) Usage
|
General Description |
This molecule has been used in the synthesis of novel metal-free organic dyes for Dye-Sensitized Solar Cells reaching conversion efficiencies of 6.23%. |
InChI:InChI=1/C7H4OS2/c8-4-5-3-7-6(10-5)1-2-9-7/h1-4H
31486-86-9 Relevant articles
Synthesis and evaluation of simple molecule as a co-adsorbent dye for highly efficient co-sensitized solar cells
Zhu, Shengbo,An, Zhongwei,Sun, Xiao,Wu, Zhisheng,Chen, Xinbing,Chen, Pei
, p. 85 - 92 (2015)
One simple-structure dye and two bulky d...
A systematic study of the structure-property relationship of a series of nonlinear optical (NLO) julolidinyl-based chromophores with a thieno[3,2-b]thiophene moiety
Zhang, Airui,Xiao, Hongyan,Cong, Shengyu,Zhang, Maolin,Zhang, Hua,Bo, Shuhui,Wang, Qi,Zhen, Zhen,Liu, Xinhou
, p. 370 - 381 (2015)
A series of nonlinear optical (NLO) chro...
Rigid triarylamine-based D-A-π-A structural organic sensitizers for solar cells: The significant enhancement of open-circuit photovoltage with a long alkyl group
Hu, Xiaohao,Cai, Shengyun,Tian, Guojian,Li, Xin,Su, Jianhua,Li, Jing
, p. 22544 - 22553 (2013)
Five new organic D-A-π-A sensitizers DIA...
Thieno[3,2-b]thiophene fused BODIPYs: synthesis, near-infrared luminescence and photosensitive properties
Sun, Yijuan,Qu, Zhirong,Zhou, Zhikuan,Gai, Lizhi,Lu, Hua
, p. 3617 - 3622 (2019)
The fusion of π-sufficient heteroaryl mo...
Small isomeric push-pull chromophores based on thienothiophenes with tunable optical (non)linearities
Podlesny, Jan,Pytela, Old?ich,Klikar, Milan,Jelínková, Veronika,Kityk, Iwan V.,Ozga, Katarzyna,Jedryka, Jaroslaw,Rudysh, Myron,Bure?, Filip
, p. 3623 - 3634 (2019/04/14)
Fourteen new D-π-A push-pull chromophore...
Discovery of Potent and Orally Bioavailable GPR40 Full Agonists Bearing Thiophen-2-ylpropanoic Acid Scaffold
Li, He,Huang, Qi,Chen, Cheng,Xu, Bin,Wang, He-Yao,Long, Ya-Qiu
, p. 2697 - 2717 (2017/04/21)
The free fatty acid receptor GPR40 is pr...
Synthesis, Fluorescence, and Two-Photon Absorption Properties of Push–Pull 5-Arylthieno[3,2-b]thiophene Derivatives
Manuela,Raposo,Herbivo, Cyril,Hugues, Vincent,Clermont, Guillaume,Castro, M. Cidália R.,Comel, Alain,Blanchard-Desce, Mireille
, p. 5263 - 5273 (2016/11/13)
Three series of novel push–pull 5-arylth...
31486-86-9 Process route
-
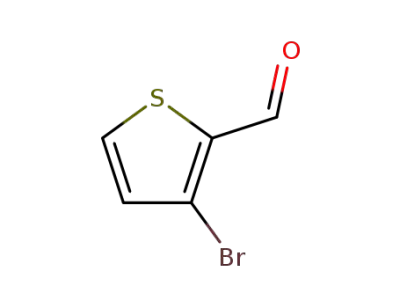
-
930-96-1
3-bromo-2-thiophenecarboxaldehyde

-
![thieno[3,2-b]thiophene-2-carbaldehyde](/upload/2026/1/1247bd50-39a2-4f11-828d-5f0fd490d179.png)
-
31486-86-9
thieno[3,2-b]thiophene-2-carbaldehyde
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 6 steps
1: 17.6 g / pyridinium tosylate / toluene / 1 h / Heating
3: p-toluenesulfonic acid hydrate / acetone / 1 h
4: 36.93 g / piperidine, acetic acid / benzene / 5 h / Heating
5: LiAlH4 / diethyl ether / 3 h / Ambient temperature
6: 23.30 g / pyridinium chlorochromate / CH2Cl2 / 2.5 h
With
piperidine; lithium aluminium tetrahydride; pyridinium p-toluenesulfonate; toluene-4-sulfonic acid; acetic acid; pyridinium chlorochromate;
In
diethyl ether; dichloromethane; acetone; toluene; benzene;
|
|
|
Multi-step reaction with 3 steps
1: potassium carbonate / N,N-dimethyl-formamide / 72 h / 60 °C
2: lithium aluminium tetrahydride / diethyl ether / 5 h / 0 - 20 °C
3: pyridinium chlorochromate / dichloromethane / 2.5 h / 20 °C
With
lithium aluminium tetrahydride; potassium carbonate; pyridinium chlorochromate;
In
diethyl ether; dichloromethane; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 4 steps
1: potassium carbonate / N,N-dimethyl-formamide / 3 h / 25 °C
2: lithium hydroxide; water / tetrahydrofuran / 4 h / 100 °C
3: copper(l) iodide; N,N,N,N,-tetramethylethylenediamine / 1-methyl-pyrrolidin-2-one / 1 h / 220 °C
4: trichlorophosphate / 12 h / 25 - 60 °C
With
copper(l) iodide; N,N,N,N,-tetramethylethylenediamine; water; potassium carbonate; lithium hydroxide; trichlorophosphate;
In
tetrahydrofuran; 1-methyl-pyrrolidin-2-one; N,N-dimethyl-formamide;
4: |Vilsmeier-Haack Formylation;
|
-
![thieno[3,2-b]thiophene](/upload/2026/1/bfb707b3-dc24-4dad-94c3-0ea503cb78d8.png)
-
251-41-2
thieno[3,2-b]thiophene

-
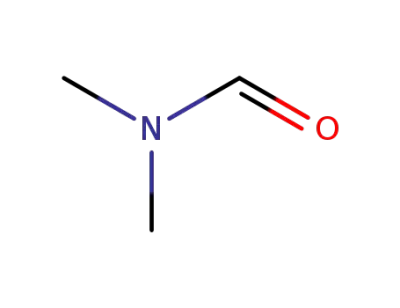
-
68-12-2,33513-42-7
N,N-dimethyl-formamide

-
![thieno[3,2-b]thiophene-2-carbaldehyde](/upload/2026/1/1247bd50-39a2-4f11-828d-5f0fd490d179.png)
-
31486-86-9
thieno[3,2-b]thiophene-2-carbaldehyde
| Conditions | Yield |
|---|---|
|
With
trichlorophosphate;
at 25 - 60 ℃;
for 12h;
|
93% |
|
With
trichlorophosphate;
In
1,2-dichloro-ethane;
at 70 ℃;
for 4h;
Inert atmosphere;
|
86% |
|
thieno[3,2-b]thiophene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 - -10 ℃;
for 1.5h;
Inert atmosphere;
N,N-dimethyl-formamide;
In
tetrahydrofuran; hexane;
at -78 ℃;
Reagent/catalyst;
Inert atmosphere;
|
85% |
|
thieno[3,2-b]thiophene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 6h;
Inert atmosphere;
N,N-dimethyl-formamide;
In
tetrahydrofuran; hexane;
at 20 ℃;
for 16h;
Inert atmosphere;
|
82% |
|
With
trichlorophosphate;
In
1,2-dichloro-ethane;
at 0 - 20 ℃;
|
75% |
|
With
trichlorophosphate;
In
1,2-dichloro-ethane;
at 0 ℃;
Reflux;
|
74.98% |
|
With
trichlorophosphate;
In
1,2-dichloro-ethane;
at 0 - 80 ℃;
for 5h;
Inert atmosphere;
|
67% |
|
With
trichlorophosphate;
In
1,2-dichloro-ethane;
at 0 - 70 ℃;
Inert atmosphere;
|
61% |
|
thieno[3,2-b]thiophene; N,N-dimethyl-formamide;
With
trichlorophosphate;
In
1,2-dichloro-ethane;
at 90 ℃;
for 48h;
Cooling with ice;
With
water;
In
1,2-dichloro-ethane;
Cooling with ice;
|
59% |
|
With
n-butyllithium;
In
hexane;
at 0 ℃;
for 1.33333h;
|
|
|
With
trichlorophosphate;
at 0 - 100 ℃;
for 4h;
|
|
|
With
lithium diisopropyl amide;
|
31486-86-9 Upstream products
-
127025-34-7
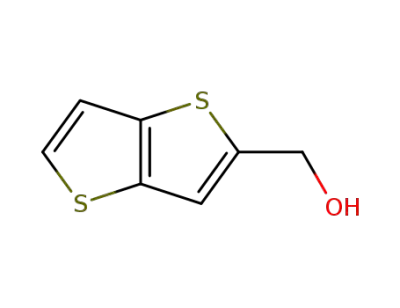
2-(hydroxymethyl)thieno<3,2-b>thiophene
-
930-96-1

3-bromo-2-thiophenecarboxaldehyde
-
1723-29-1
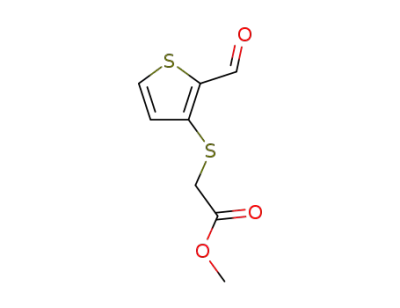
methyl 3-<2-(2-formylyhiophene-3-yl)>-3-thiapropionate
-
56857-02-4
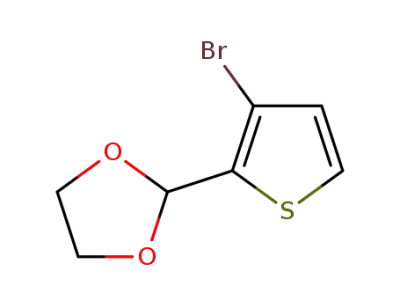
2-(3-bromo-thien-2-yl)-1,3-dioxolane
31486-86-9 Downstream products
-
392662-57-6
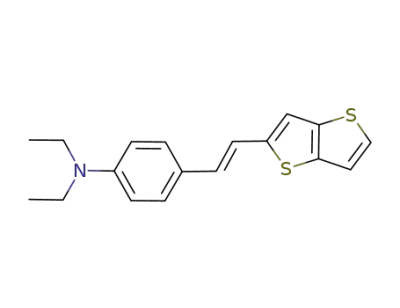
N,N-diethyl-4-((E)-2-(thieno[3,2-b]thien-2-yl)vinyl)aniline
-
1092363-80-8
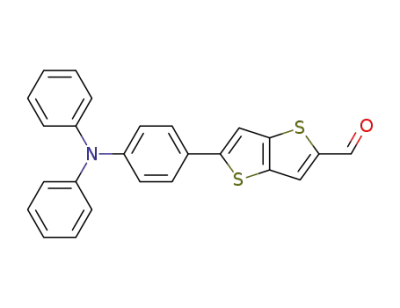
5-(4-(diphenylamino)phenyl)thieno[3,2-b]thiophene-2-carbaldehyde
-
1346644-07-2
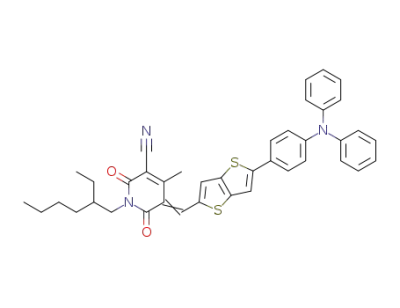
5-((5-(4-(diphenylamino)phenyl)thieno[3,2-b]thiophene-2-yl)methylene)-1-(2-ethylhexyl)-4-methyl-2,6-dioxo-1,2,5,6-tetrahydropyridine-3-carbonitrile
-
84466-27-3
2-styrylthieno[3,2-b]thiophene
Relevant Products
-
Propanesulphonyl chloride
CAS:10147-36-1
-
3-Chloro-2-fluorobroMobenzene
CAS:144584-65-6
-
1,1'-Bis(diisopropylphosphino)ferrocene
CAS:97239-80-0