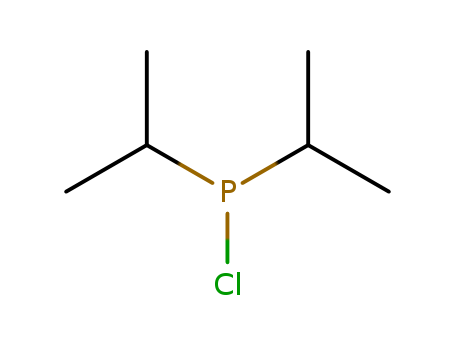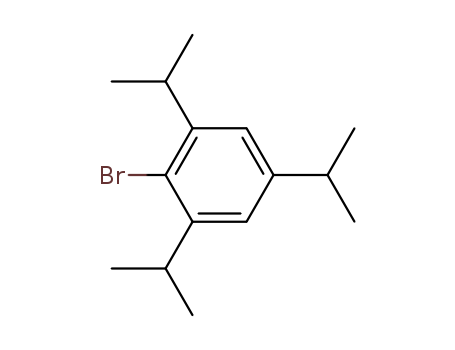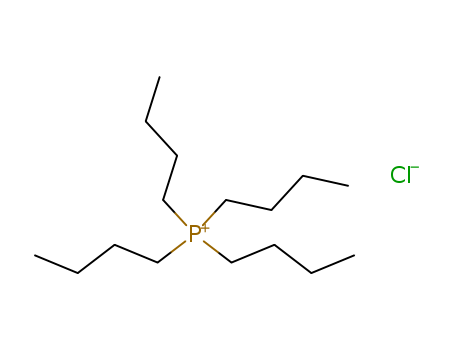
2304-30-5
- Product Name:Tetrabutylphosphonium chloride
- Molecular Formula:C16H36ClP
- Purity:99%
- Molecular Weight:294.889
Product Details;
CasNo: 2304-30-5
Molecular Formula: C16H36ClP
Appearance: clear to yellowish liquid
factory and manufacture 2304-30-5 Tetrabutylphosphonium chloride lonic liquid
- Molecular Formula:C16H36ClP
- Molecular Weight:294.889
- Appearance/Colour:clear to yellowish liquid
- Vapor Pressure:0.018Pa at 25℃
- Melting Point:62-66 °C
- Refractive Index:1.5
- Boiling Point:344.8℃[at 101 325 Pa]
- Flash Point:4°C (39°F)
- PSA:13.59000
- Density:0.978[at 20℃]
- LogP:3.20840
Tetrabutylphosphonium chloride(Cas 2304-30-5) Usage
|
General Description |
This product has been enhanced for catalytic efficiency. |
InChI:InChI=1/C16H36P.ClH/c1-5-9-13-17(14-10-6-2,15-11-7-3)16-12-8-4;/h5-16H2,1-4H3;1H/q+1;
2304-30-5 Relevant articles
A comparison of the oxidation of lignin model compounds in conventional and ionic liquid solvents and application to the oxidation of lignin
Yao, Soledad G.,Meier, Mark S.,Pace, Robert B.,Crocker, Mark
, p. 104742 - 104753 (2016)
The oxidation of lignin model compounds ...
Microwave synthesis of microstructured and nanostructured metal chalcogenides from elemental precursors in phosphonium ionic liquids
Ding, Kunlun,Lu, Hong,Zhang, Yichi,Snedaker, Matthew L.,Liu, Deyu,Maci-Agull, Juan Antonio,Stucky, Galen D.
, p. 15465 - 15468 (2014)
We describe a general approach for the s...
Comparative study of inclusion complexation of tetraalkylphosphonium and ammonium salts with cucurbit[7]uril
Hagiwara, Seiya,Hanaya, Tadashi,Matsumoto, Yuki,Sueishi, Yoshimi
, (2020/07/13)
Inclusion complexation of tetraalkylphos...
METHOD OF PRODUCING VINYL CHLORIDE
-
Paragraph 0030, (2020/01/27)
A method of producing vinyl chloride is ...
Synthesis of Functional Monosilanes by Disilane Cleavage with Phosphonium Chlorides
Santowski, Tobias,Sturm, Alexander G.,Lewis, Kenrick M.,Felder, Thorsten,Holthausen, Max C.,Auner, Norbert
supporting information, p. 3809 - 3815 (2019/02/13)
The Müller–Rochow direct process (DP) fo...
Effects of charge balance and hydrophobicity of the surface of cytochrome: C on the distribution behaviour in an ionic liquid/buffer biphasic system
Ikeda, Kazuma,Fujita, Kyoko,Ohno, Hiroyuki,Nakamura, Nobuhumi
, p. 7337 - 7341 (2019/08/15)
Factors contributing to the different di...
2304-30-5 Process route
-
-
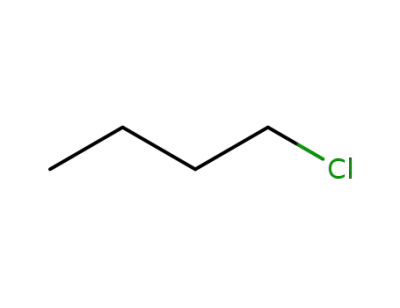
109-69-3
n-Butyl chloride

-
-
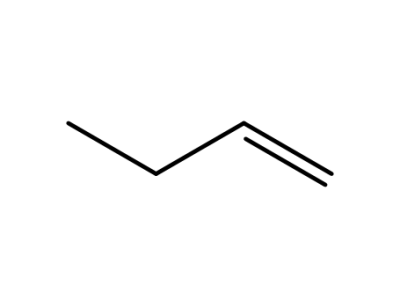
106-98-9,9003-28-5
1-butylene

-
-
2304-30-5
tetra-n-butylphosphonium chloride
| Conditions | Yield |
|---|---|
|
With
tributylphosphine;
In
(2)H8-toluene;
at 220 ℃;
for 16h;
Schlenk technique;
Inert atmosphere;
Sealed tube;
|
-

-
109-69-3
n-Butyl chloride

-

-
998-40-3
tributylphosphine

-

-
2304-30-5
tetra-n-butylphosphonium chloride
| Conditions | Yield |
|---|---|
|
In
diethyl ether;
Reflux;
|
89% |
|
at 115 ℃;
for 72h;
|
|
|
at 50 - 95 ℃;
for 192h;
Inert atmosphere;
|
|
|
at 105 ℃;
for 72h;
|
2304-30-5 Upstream products
-
14518-69-5
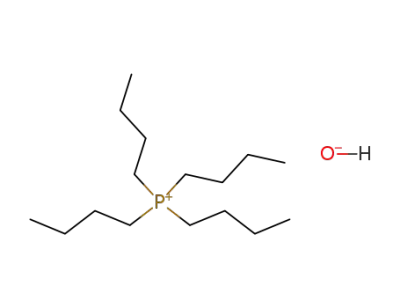
tetra-n-butylphosphonium hydroxide
-
109-69-3

n-Butyl chloride
-
998-40-3

tributylphosphine
-
693-04-9
butyl magnesium bromide
2304-30-5 Downstream products
-
1888-71-7
perchloropropene
-
455-16-3
p-toluenesulfonyl fluoride
-
71715-84-9
2,4,6-tris(o-aminophenylthiomethyl)mesitylene
-
52645-53-1
permethrin
Relevant Products
-
Chlorodiisopropylphosphine
CAS:40244-90-4
-
2-BroMo-1,3,5-triisopropylbenzene
CAS:21524-34-5
-
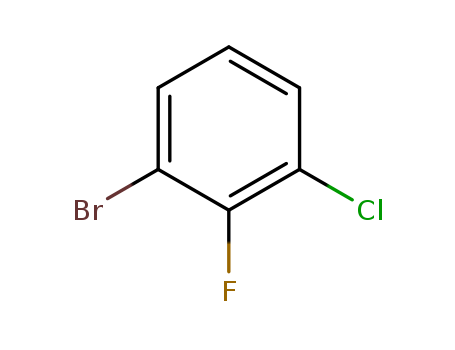
3-Chloro-2-fluorobroMobenzene
CAS:144584-65-6