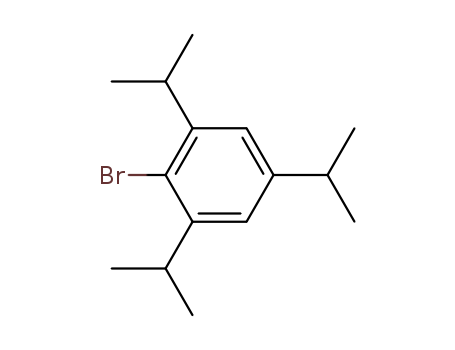
21524-34-5
- Product Name:2-BroMo-1,3,5-triisopropylbenzene
- Molecular Formula:C15H23Br
- Purity:99%
- Molecular Weight:283.252
Product Details;
CasNo: 21524-34-5
Molecular Formula: C15H23Br
factory and supplier 21524-34-5 2-BroMo-1,3,5-triisopropylbenzene in stock
- Molecular Formula:C15H23Br
- Molecular Weight:283.252
- Vapor Pressure:0.00252mmHg at 25°C
- Refractive Index:n20/D 1.523(lit.)
- Boiling Point:296.511 °C at 760 mmHg
- Flash Point:125.937 °C
- PSA:0.00000
- Density:1.107 g/cm3
- LogP:5.81930
1-BROMO-2,4,6-TRIISOPROPYLBENZENE(Cas 21524-34-5) Usage
|
Synthesis Reference(s) |
Synthesis, p. 621, 1976 DOI: 10.1055/s-1976-24146 |
InChI:InChI=1/C15H23Br/c1-9(2)12-7-13(10(3)4)15(16)14(8-12)11(5)6/h7-11H,1-6H3
21524-34-5 Relevant articles
Stepwise mechanism for the bromination of arenes by a hypervalent iodine reagent
Arrieta, Ana,Cossío, Fernando P.,Granados, Albert,Shafir, Alexandr,Vallribera, Adelina
, p. 2142 - 2150 (2020/03/11)
A mild, metal-free bromination method of...
Enantioselective organocatalytic fluorination-induced Wagner-Meerwein rearrangement
Romanov-Michailidis, Fedor,Guénée, Laure,Alexakis, Alexandre
supporting information, p. 9266 - 9270 (2013/09/12)
Cracked under strain: Strained allylic c...
Reactivity and synthetic utility of 1-(arenesulfonyloxy) benziodoxolones
Muraki, Takahito,Togo, Hideo,Yokoyama, Masataka
, p. 2883 - 2889 (2007/10/03)
The reactivity and synthetic use of 1-(a...
Synthetic use of 1-(p-toluenesulfonyloxy)-1,2-benziodoxol-3(1H)-one: Iodination of aromatic rings
Muraki, Takahito,Togo, Hideo,Yokoyama, Masataka
, p. 286 - 288 (2007/10/03)
Treatment of various aromatic compounds ...
21524-34-5 Process route
-
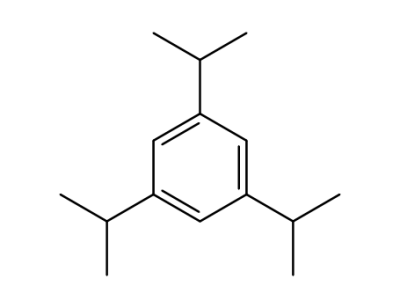
-
717-74-8
1,3,5-triisopropyl benzene

-
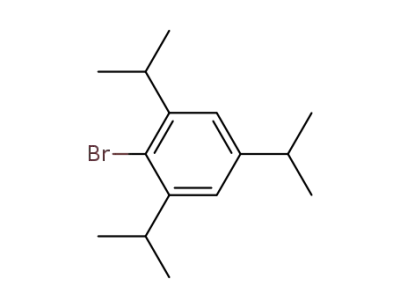
-
21524-34-5
2,4,6-triisopropyl-1-bromobenzene
| Conditions | Yield |
|---|---|
|
With
1-(p-toluenebenzenesulfonyloxy)-1,2-benziodoxol-3(1H)-on; tetrabutylammomium bromide;
In
acetonitrile;
for 16h;
Product distribution;
Ambient temperature;
other reagents (trivalent iodine compounds + LiBr or Bu4NBr), other reagent ratio;
|
84% |
|
With
tetrabutylammomium bromide; 1-(p-methylbenzenesulfonyloxy)-1,2-benziodoxol-3(1H)-one;
In
acetonitrile;
for 16h;
Ambient temperature;
|
84% |
|
With
bis(trifluoroacetoxy)iodobencene; trimethylsilyl bromide;
In
dichloromethane;
at 20 ℃;
Solvent;
Inert atmosphere;
|
81% |
|
With
1-(p-methylbenzenesulfonyloxy)-1,2-benziodoxol-3(1H)-one; lithium bromide;
In
acetonitrile;
for 18h;
Ambient temperature;
|
79% |
|
bromination;
|
78% |
|
With
tetrachloromethane; bromine; iron;
at 0 ℃;
Lichtausschluss;
|
|
|
With
bromine; iron;
|
|
|
With
bromine;
In
N,N-dimethyl-formamide;
|
|
|
With
bromine; sodium ethanolate; iron; hydroquinone;
Yield given. Multistep reaction;
1) 1 h, 20 deg C, CCl4, 2) EtOH, 2 h, reflux; 18 h, 20 deg C;
|
|
|
With
bromine;
In
chloroform;
at 0 - 20 ℃;
for 2h;
Inert atmosphere;
|
-
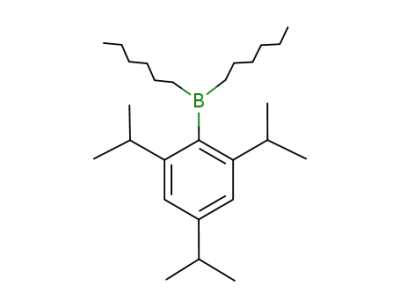
-
148163-04-6
(2,4,6-triisopropylphenyl)dihexylborane

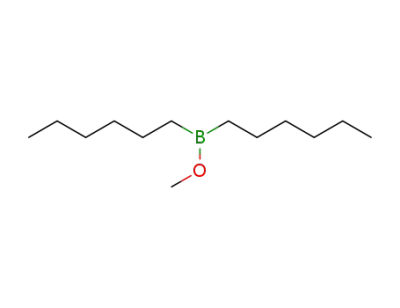
-
-
2344-22-1
methyl dihexylborinic ester

-
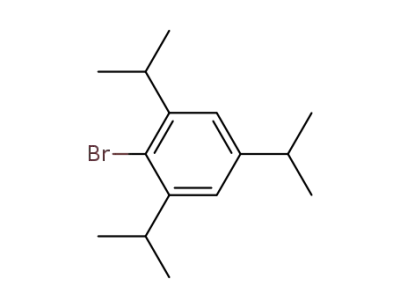
-
21524-34-5
2,4,6-triisopropyl-1-bromobenzene
| Conditions | Yield |
|---|---|
|
With
methanol; water; bromine;
Bromination of starting compd.;
|
91% |
21524-34-5 Upstream products
-
717-74-8

1,3,5-triisopropyl benzene
-
56-23-5
tetrachloromethane
-
7726-95-6
bromine
-
148163-04-6

(2,4,6-triisopropylphenyl)dihexylborane
21524-34-5 Downstream products
-
49623-71-4
2,4,6-triisopropylbenzoic acid
-
137144-19-5
2,4,6-triisopropylphenyl 9-xanthenyl ketone
-
53356-73-3
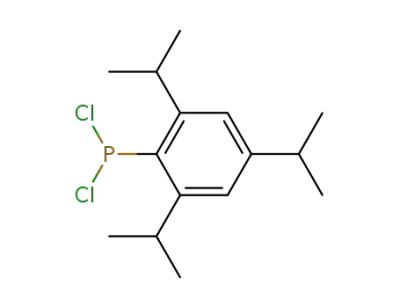
dichloro(2,4,6-tri-isopropylphenyl)phosphane
-
182227-72-1
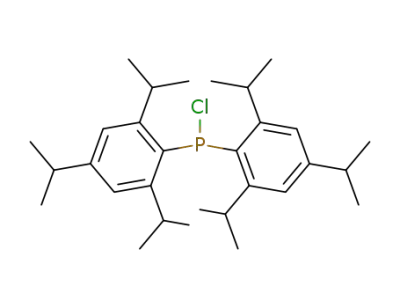
bis(2,4,6-tri-isopropylphenyl)phosphinous chloride
Relevant Products
-
5-Bromo-2-fluoro-m-xylene
CAS:99725-44-7
-
Tetrabutylphosphonium chloride
CAS:2304-30-5