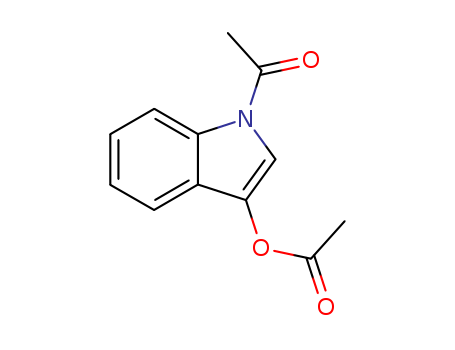
16800-67-2
- Product Name:1,3-DIACETOXYINDOLE
- Molecular Formula:C12H11NO3
- Purity:99%
- Molecular Weight:217.224
Product Details;
CasNo: 16800-67-2
Molecular Formula: C12H11NO3
Appearance: Light brown solid
factory and supplier 16800-67-2 1,3-DIACETOXYINDOLE in stock
- Molecular Formula:C12H11NO3
- Molecular Weight:217.224
- Appearance/Colour:Light brown solid
- Vapor Pressure:6.21E-05mmHg at 25°C
- Melting Point:80 °C
- Refractive Index:1.578
- Boiling Point:345.3 °C at 760 mmHg
- Flash Point:162.6 °C
- PSA:48.30000
- Density:1.21 g/cm3
- LogP:2.22670
1,3-DIACETOXYINDOLE(Cas 16800-67-2) Usage
InChI:InChI=1/C12H11NO3/c1-8(14)13-7-12(16-9(2)15)10-5-3-4-6-11(10)13/h3-7H,1-2H3
16800-67-2 Relevant articles
Orally Effective Aminoalkyl 10H-Indolo[3,2-b]quinoline-11-carboxamide Kills the Malaria Parasite by Inhibiting Host Hemoglobin Uptake
Mudududdla, Ramesh,Mohanakrishnan, Dinesh,Bharate, Sonali S.,Vishwakarma, Ram A.,Sahal, Dinkar,Bharate, Sandip B.
, p. 2581 - 2598 (2018)
A series of indolo[3,2-b]quinoline-C11-c...
Pharmacological evaluation of novel PKR inhibitor indirubin-3-hydrazone in-vitro in cardiac myocytes and in-vivo in wistar rats
Udumula, Mary Priyanka,Bhat, Audesh,Mangali, Sureshbabu,Kalra, Jaspreet,Dhar, Indu,Sriram, Dharamrajan,Dhar, Arti
, p. 85 - 96 (2018)
Aims: Double stranded protein kinase R c...
A concise synthesis of the DNA-intercalating and antimalarial alkaloid cryptolepine and its fluorescence behaviour in solvents of different polarities
Lai, Tapan Kumar,Chatterjee, Asima,Banerji, Julie,Sarkar, Deboleena,Chattopadhyay, Nitin
, p. 1975 - 1983 (2008)
A microwave-induced rapid and facile syn...
Synthesis and biological evaluation of indoloquinoline alkaloid cryptolepine and its bromo-derivative as dual cholinesterase inhibitors
Nuthakki, Vijay K.,Mudududdla, Ramesh,Sharma, Ankita,Kumar, Ajay,Bharate, Sandip B.
, (2019)
Alkaloids have always been a great sourc...
-
Tighineanu,E. et al.
, p. 1887 - 1890 (1978)
-
Phenylimino Indolinone: A Green-Light-Responsive T-Type Photoswitch Exhibiting Negative Photochromism
Buma, Wybren Jan,Crespi, Stefano,Di Donato, Mariangela,Doria, Sandra,Feringa, Ben L.,Hilbers, Michiel F.,Kiss, Ferdinand L.,Simeth, Nadja A.,Stindt, Charlotte N.,Szymański, Wiktor,Toyoda, Ryojun,Wesseling, Sammo
supporting information, p. 25290 - 25295 (2021/10/25)
Imines are photoaddressable motifs usefu...
Construction of Oxepino[3,2-b]indoles via [4+3] Annulation of 2-Ylideneoxindoles with Crotonate-Derived Sulfur Ylides
Fei, Xing-Hai,Guan, Xiang,He, Bin,Li, Zong-Qin,Wang, Da-Peng,Yang, Fen-Fen,Yang, Yuan-Yong,Zhao, Yong-Long,Zhou, Meng
supporting information, p. 3018 - 3024 (2021/06/26)
A [4+3] annulation of 2-ylideneoxindoles...
Indirubin Derivatives as Dual Inhibitors Targeting Cyclin-Dependent Kinase and Histone Deacetylase for Treating Cancer
An, Jianxiong,Cao, Zhuoxian,Gu, Zhicheng,He, Bin,Li, Yan,Li, Yongjun,Lin, Hening,Lin, Shuxian,Liu, Ting,Wang, Jie,Wang, Pan,Yang, Fenfen,Zhao, Yonglong
, p. 15280 - 15296 (2021/10/25)
To utilize the unique scaffold of a natu...
16800-67-2 Process route
-
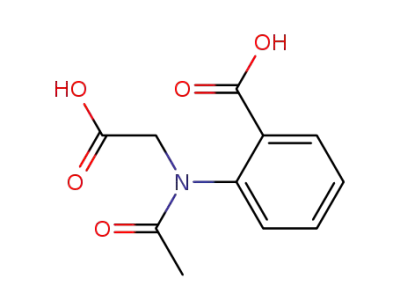
-
16851-69-7
2-(N-(carboxymethyl)acetamido)benzoic acid

-
-
108-24-7
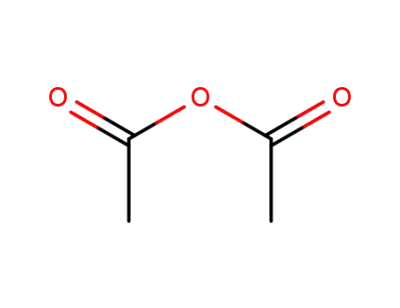
acetic anhydride

-
-
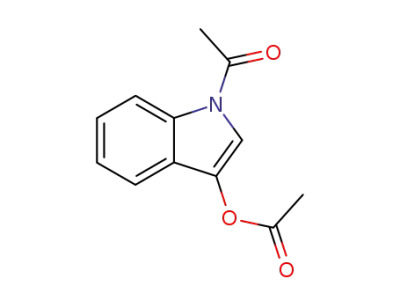
16800-67-2
N,O-diacetylindoxyl
| Conditions | Yield |
|---|---|
|
With
pyridine;
at 55 - 60 ℃;
for 24h;
|
90% |
|
With
triethylamine;
for 0.5h;
Inert atmosphere;
Schlenk technique;
Reflux;
|
89% |
|
With
triethylamine;
In
water;
at 20 ℃;
for 0.333333h;
Heating / reflux;
|
86% |
|
With
triethylamine;
for 0.0666667h;
Microwave irradiation;
Inert atmosphere;
|
86% |
|
With
triethylamine;
at 100 ℃;
|
80% |
|
With
triethylamine;
at 100 ℃;
|
80% |
|
With
triethylamine;
Heating;
|
-
-
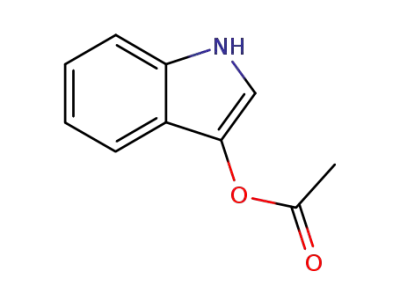
608-08-2
3-indoxyl acetate

-

-
108-24-7
acetic anhydride

-

-
16800-67-2
N,O-diacetylindoxyl
| Conditions | Yield |
|---|---|
|
With
dmap;
In
tetrahydrofuran;
at 85 ℃;
for 16h;
Inert atmosphere;
Schlenk technique;
Sealed tube;
|
93% |
|
With
triethylamine;
at 80 - 90 ℃;
for 0.5h;
|
82% |
|
With
dmap; triethylamine;
In
tetrahydrofuran;
for 3h;
Heating / reflux;
|
67% |
16800-67-2 Upstream products
-
16800-68-3
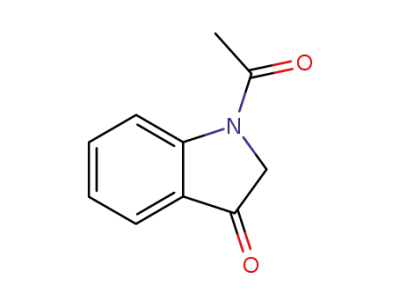
1-acetyl-2,3-dihydro-1H-indol-3-one
-
108-24-7

acetic anhydride
-
480-93-3
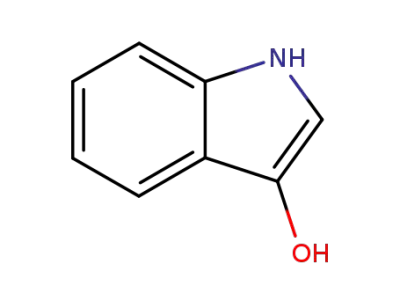
indoxyl
-
612-42-0
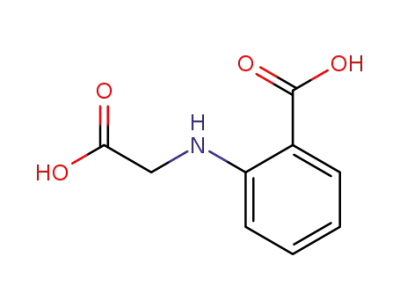
phenylglycine-o-carboxylic acid
16800-67-2 Downstream products
-
16078-30-1
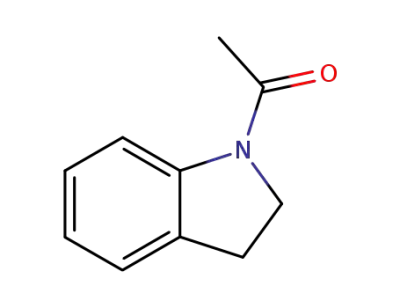
1-Acetyl-2,3-dihydro-1H-indole
-
99293-86-4
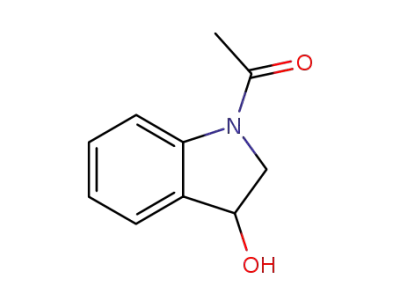
N-acetylindolin-3-ol
-
33025-60-4

1-(3-hydroxy-1H-indol-1-yl)ethanone
-
110912-08-8
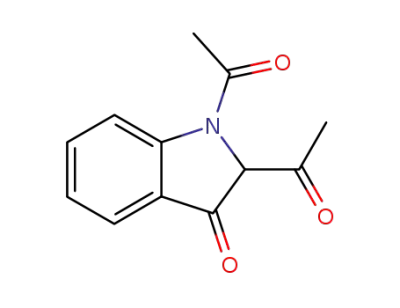
1,1'-(3-oxoindoline-1,2-diyl)bis(ethan-1-one)
Relevant Products
-
2,5-Dimethoxy-Beta-Nitrostyrene
CAS:40276-11-7
-
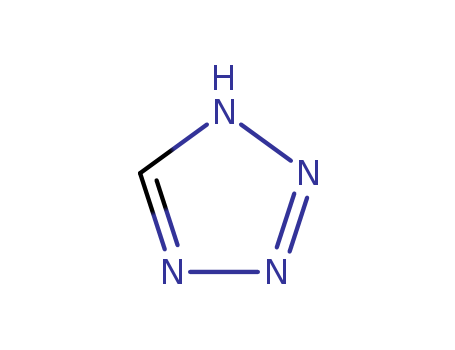
Tetrazole
CAS:288-94-8
-
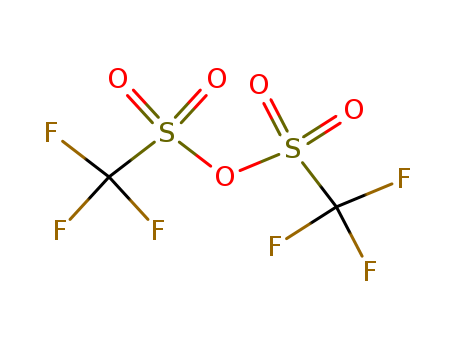
Trifluoromethanesulfonic anhydride
CAS:358-23-6