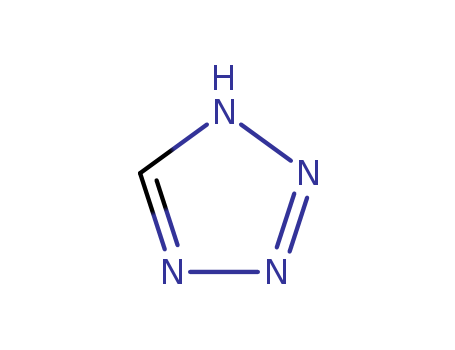
288-94-8
- Product Name:Tetrazole
- Molecular Formula:CH2N4
- Purity:99%
- Molecular Weight:70.0537
Product Details;
CasNo: 288-94-8
Molecular Formula: CH2N4
Appearance: white crystals or crystalline powder
factory and supplier 288-94-8 Tetrazole in stock
- Molecular Formula:CH2N4
- Molecular Weight:70.0537
- Appearance/Colour:white crystals or crystalline powder
- Vapor Pressure:394mmHg at 25°C
- Melting Point:156-158 °C
- Refractive Index:n20/D 1.348
- Boiling Point:220.233 °C at 760 mmHg
- PKA:4.9(at 25℃)
- Flash Point:114.573 °C
- PSA:54.46000
- Density:1.477 g/cm3
- LogP:-0.80030
Tetrazole(Cas 288-94-8) Usage
|
Preparation |
The first tetrazole synthesis was reported in 1885.Tetrazole Synthesis: Nano-TiCl4.SiO2 (0.1 g) was added to a mixture of benzonitrile (1 mmol), sodium azide (2 mmol) in DMF (5 mL) at reflux for 2 h. After completion of reaction (as monitored by TLC), the mixture was allowed to cool to room temperature, the catalyst was removed by filtration. Then by adding ice water and 4N HCl (5 mL) to the residue, a white solid was obtained. This was then washed with cold chloroform. This simple procedure yielded pure tetrazole with good yields. |
|
Health Hazard |
Fire may produce irritating, corrosive and/or toxic gases. |
|
Fire Hazard |
MAY EXPLODE AND THROW FRAGMENTS 1600 meters (1 MILE) OR MORE IF FIRE REACHES CARGO. |
|
Purification Methods |
Crystallise the tetrazole from EtOH and sublime it under high vacuum at ca 120o (care should be taken due to possible EXPLOSION). [Beilstein 26 H 346, 26 I 108, 26 II 196, 26 III/IV 1652.] |
|
General Description |
Tetrazole is a heterocyclic compound with the molecular formula CH2N4, existing primarily in the 1H-tautomeric form in the gas phase, as confirmed by thermodynamic studies. It exhibits a reversible solid-to-solid transition around 230–245 K and has a fusion temperature of 430 K with an enthalpy of fusion of 18.0 kJ·mol-1. The sublimation enthalpy of crystalline tetrazole is approximately 88.25 kJ·mol-1 at 350 K, and its thermodynamic properties have been extensively characterized in both solid and gaseous states. Additionally, tetrazole serves as a versatile synthetic intermediate, functioning as an efficient leaving group in organic reactions, such as the microwave-assisted synthesis of substituted pyrimidines. It also plays a role in coordination chemistry, forming complexes with transition metals like iron(II) in spin-crossover systems. Furthermore, tetrazole derivatives, such as sodium 5-chlorotetrazolate, can be synthesized via chlorination, demonstrating distinct thermal decomposition behaviors compared to their parent compounds. |
|
Application |
1H-Tetrazole is used as a bioisostere for the carboxylate group. It is also used as coupling reagent for preparation of polynucleotides. |
|
Definition |
ChEBI: 1H-tetrazole is a tetrazole tautomer where the proton is located on the 1st position. It is a tetrazole and a one-carbon compound. It is a tautomer of a 2H-tetrazole and a 5H-tetrazole. |
InChI:InChI=1/CH2N4/c1-2-4-5-3-1/h1H2
288-94-8 Relevant articles
Mild and Catalyst-Free Microwave-Assisted Synthesis of 4,6-Disubstituted 2-Methylthiopyrimidines - Exploiting Tetrazole as an Efficient Leaving Group
Thomann, Andreas,Eberhard, Jens,Allegretta, Giuseppe,Empting, Martin,Hartmann, Rolf W.
, p. 2606 - 2610 (2015)
Typically, 4,6-disubstituted 2-thiomethy...
Thermodynamic properties and tautomerism of tetrazole
Kabo, G. J.,Kozyro, A. A.,Krasulin, A. P.,Sevruk, V. M.,Ivashkevich, L. S.
, p. 485 - 493 (1993)
The results of a study of tetrazole in d...
Evidence of Ligand Elasticity Occurring in Temperature-, Light-, and Pressure-Induced Spin Crossover in 1D Coordination Polymers [Fe(3ditz)3]X2 (X = ClO4–, BF4–)
Weselski, Marek,Ksi??ek, Maria,Kusz, Joachim,Bia?ońska, Agata,Paliwoda, Damian,Hanfland, Michael,Rudolf, Miko?aj F.,Ciunik, Zbigniew,Bronisz, Robert
, p. 1171 - 1179 (2017)
The complexes [M(3ditz)3]X2 [X = ClO4–, ...
Synthesis and Characterization of Sodium 5-Chlorotetrazolate Dihydrate by Chlorination of 1H-Tetrazole
Wang, Xiaojun,Liu, Jiping,Wang, Dong,Bi, Xiaolu,Zhao, Wei
, p. 631 - 635 (2015)
A convenient, simple work-up procedure a...
SNAr azidation of phenolic functions utilizing diphenyl phosphorazidate
Ishihara, Kotaro,Shioiri, Takayuki,Matsugi, Masato
supporting information, (2019/12/27)
A useful method for the synthesis of ary...
2-Methyl-substituted monotetrazoles in copper(ii) perchlorate complexes: manipulating coordination chemistry and derived energetic properties
Zeisel, Lukas,Szimhardt, Norbert,Wurzenberger, Maximilian H. H.,Klap?tke, Thomas M.,Stierstorfer, J?rg
supporting information, p. 609 - 616 (2019/01/10)
A proposed correlation between coordinat...
Microwave alkylation of lithium tetrazolate
Müller, Danny,Knoll, Christian,Weinberger, Peter
, p. 131 - 137 (2017/01/17)
Abstract: N1-substituted tetrazoles are ...
288-94-8 Process route
-
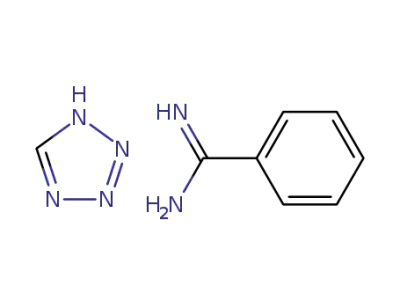
-
benzamidine tetrazole salt

-
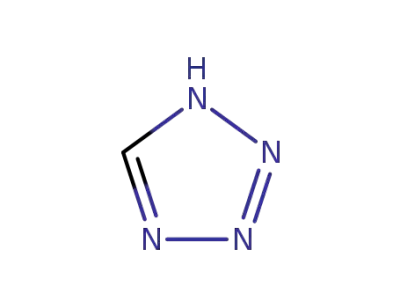
-
288-94-8
1H-tetrazole

-
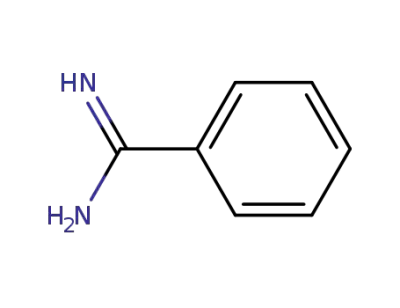
-
618-39-3
benzamidin
| Conditions | Yield |
|---|---|
|
In
methanol; acetonitrile;
Equilibrium constant;
|
-
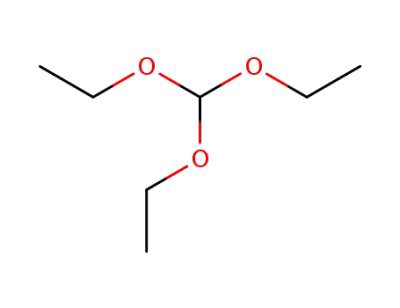
-
122-51-0
orthoformic acid triethyl ester

-

-
288-94-8
1H-tetrazole
| Conditions | Yield |
|---|---|
|
With
sodium azide; ammonium chloride; acetic acid;
at 90 ℃;
for 10h;
|
91.55% |
|
orthoformic acid triethyl ester;
With
hydrogenchloride; sodium azide; ammonia; pyridine hydrochloride; acetic acid;
at 90 ℃;
for 12h;
With
water; sodium hydroxide;
at 20 ℃;
|
71% |
|
With
sodium azide; ammonium chloride; acetic acid;
at 95 ℃;
for 18h;
|
60.5% |
|
With
sodium azide; ammonium chloride; acetic acid;
at 80 ℃;
for 16h;
|
51% |
|
With
sodium azide; ammonium chloride;
|
|
|
With
sodium azide; ammonium chloride; acetic acid;
at 80 ℃;
for 10h;
|
288-94-8 Upstream products
-
66924-15-0
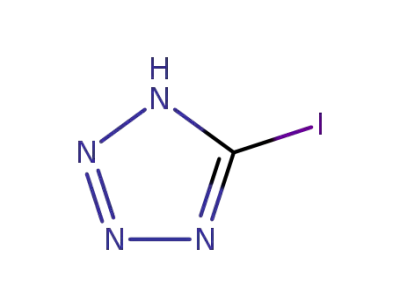
5-iodo-1H-tetrazole
-
141-52-6
sodium ethanolate
-
4418-61-5
5-aminotetrazole
-
14213-13-9
4-tetrazol-1-yl-phenylamine
288-94-8 Downstream products
-
16681-77-9
1-methyl-1,2,3,4-tetrazole
-
965-04-8
N,N'-dibenzoylurea
-
787-84-8
N'-benzoylbenzohydrazide
-
92614-86-3
3-(1H-tetrazol-1-yl)propanoic acid
Relevant Products
-
2,5-Dimethoxy-Beta-Nitrostyrene
CAS:40276-11-7
-
Pentafluorophenol
CAS:771-61-9
-
1,3-DIACETOXYINDOLE
CAS:16800-67-2