492-97-7
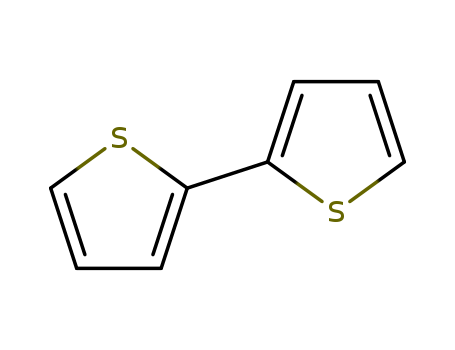
- Product Name:2,2'-Bithiophene
- Molecular Formula:C8H6S2
- Purity:99%
- Molecular Weight:166.268
Product Details;
CasNo: 492-97-7
Molecular Formula: C8H6S2
Appearance: white to light yellow crystal powder
factory and supplier 492-97-7 2,2'-Bithiophene in stock
- Molecular Formula:C8H6S2
- Molecular Weight:166.268
- Appearance/Colour:white to light yellow crystal powder
- Melting Point:32-33 °C(lit.)
- Refractive Index:1.6210 (estimate)
- Boiling Point:259.999 °C at 760 mmHg
- Flash Point:76.261 °C
- PSA:56.48000
- Density:1.244 g/cm3
- LogP:3.47660
492-97-7 Relevant articles
Desorption/ionization on self-assembled monolayer surfaces (DIAMS)
Sanguinet,Aleveque,Blanchard,Dias,Levillain,Rondeau
, p. 830 - 833 (2006)
-
Solution processable alternating oligothiophene-PEO-block-copolymers: Synthesis and evidence for solvent dependent aggregation
Kilbinger,Feast
, p. 1777 - 1784 (2000)
A new route to oligothiophene-PEO-block-...
Receptor- And ligand-based study on novel 2,2′-bithienyl derivatives as non-peptidic AANAT inhibitors
Lepailleur, Alban,Lemaitre, Stephane,Feng, Xiao,Santos, Jana Sopkova-De Oliveira,Delagrange, Philippe,Boutin, Jean,Renard, Pierre,Bureau, Ronard,Rault, Sylvain
, p. 446 - 460 (2010)
Arylalkylamine N-acetyl transferase (ser...
Reactions of lithiated aromatic heterocycles with carbon monoxide
Garcia Linares, Guadalupe E.,Nudelman, Norma S.
, p. 569 - 576 (2003)
The reaction of lithium derivatives of a...
Laser flash photolysis study on the photoinduccd reactions of 3,3′-bridged bithiophenes
Fujitsuka, Mamoru,Sato, Tadatake,Sezaki, Fumiyasu,Tanaka, Kazuyoshi,Watanabe, Akira,Ito, Osamu
, p. 3331 - 3337 (1998)
Photophysical properties and photoinduce...
Influence of donor–acceptor conjugation between thiophene-phthalazinone structures containing Sp3 C?N bond on the frontier orbital levels and optic–electronic properties
Han, Jianhua,Cheng, ShengLi,Liu, Cheng,Wang, Jinyan,Jian, Xigao
, p. 3470 - 3483 (2016)
Herein, an electron-deficient phthalazin...
Preparation and Reactions of Dichlorodithienogermoles
Ohshita, Joji,Nakamura, Masashi,Ooyama, Yousuke
, p. 5609 - 5614 (2015)
The reaction of 3,3′-dilithiobithiophene...
Far- and mid-infrared of crystalline 2,2′-bithiophene: Ab initio analysis and comparison with infrared response
Hermet,Bantignies,Rahmani,Sauvajol,Johnson,Serein
, p. 1684 - 1691 (2005)
Infrared intramolecular vibrations and l...
A novel approach to a one-pot synthesis of unsubstituted oligo(α-thiophenes)
Buzhansky, Ludmila,Feit, Ben-Ami
, p. 7523 - 7525 (2002)
Oligo(α-thiophenes) α-4T and α-8T were p...
Bithienylsilanes: Unexpected structure and reactivity
Lukevics, Edmunds,Ryabova, Victoria,Arsenyan, Pavel,Belyakov, Sergey,Popelis, Juris,Pudova, Olga
, p. 8 - 15 (2000)
5-(2,2′-Bithienyl)hydrosilanes were prep...
Copper(I)-catalysed homocoupling of organosilicon compounds: Synthesis of biaryls, dienes and diynes
Kang, Suk-Ku,Kim, Tae-Hyun,Pyun, Sung-Jae
, p. 797 - 798 (1997)
Copper(I) iodide catalyses the homocoupl...
Facile preparation of SERS-active nanogap-rich Au nanoleaves
Hong, Jin-Hyung,Hwang, Yong-Kyung,Hong, Jin-Yeon,Kim, Hee-Jin,Kim, Sung-Jin,Won, Yong Sun,Huh, Seong
, p. 6963 - 6965 (2011)
To simply reduce HAuCl4 using 2-thiophen...
SYNTHESIS OF 5-(3-PYRIDYL)-2,2'-BITHIOPHENE(SENSITIZER)
-
Page/Page column 9, (2021/02/05)
Disclosed herein is a novel simple, shor...
Electrochromic properties of pyrene conductive polymers modified by chemical polymerization
Chang, Lijing,Hou, Yanjun,Li, Rui,Ma, Yang,Miao, Shoulei,Wang, Cheng,Xu, Haoran,Zhang, Yuhang
, p. 39291 - 39305 (2021/12/27)
Pyrene is composed of four benzene rings...
Pd-catalyzed oxidative homocoupling of arylboronic acids in WEPA: A sustainable access to symmetrical biaryls under added base and ligand-free ambient conditions
Appa, Rama Moorthy,Lakshmidevi, Jangam,Naidu, Bandameeda Ramesh,Venkateswarlu, Katta
, (2021/01/11)
Symmetrical and unsymmetrical biaryls co...
Recyclable Pd2dba3/XPhos/PEG-2000 System for Efficient Borylation of Aryl Chlorides: Practical Access to Aryl Boronates
Cai, Mingzhong,Huang, Bin,Luo, Chengkai,Xu, Caifeng
, (2021/12/02)
Pd2dba3/XPhos in poly(ethylene glycol) (...
492-97-7 Process route
-
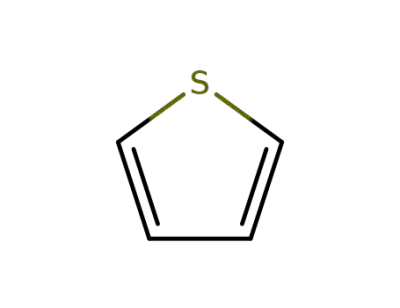
-
188290-36-0,8014-23-1,25233-34-5
thiophene

-
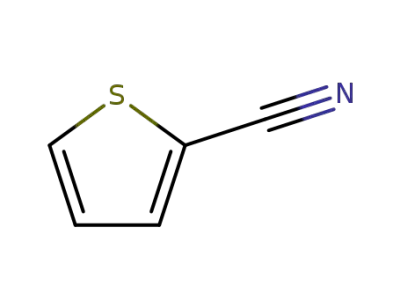
-
1003-31-2
thiophene-2-carbonitrile

-
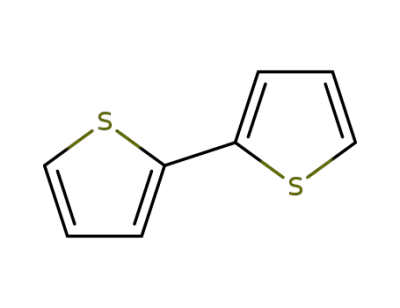
-
492-97-7
2,2'-Bithiophene
| Conditions | Yield |
|---|---|
|
With
aluminium trichloride; ethanedinitrile;
|
-
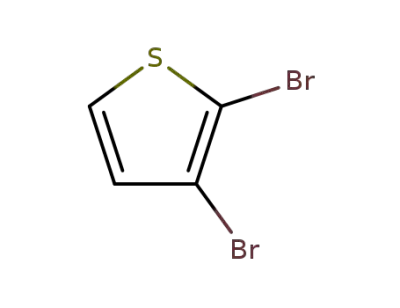
-
3140-93-0
2,3-dibromothiophen

-
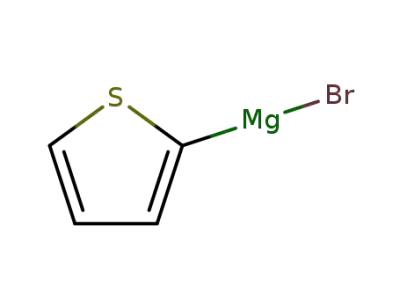
-
5713-61-1
thiophen-2-yl magnesium bromide

-
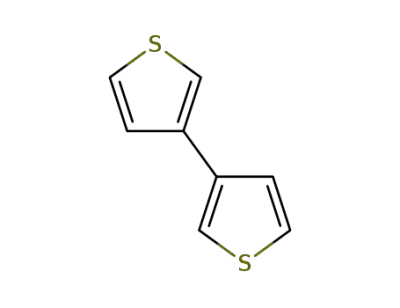
-
3172-56-3
3,3'-bithiophene

-

-
492-97-7
2,2'-Bithiophene

-
![3-bromo-[2,2’]bithiophenyl](/upload/2026/1/91385831-0a2f-4577-880f-42a1a7ef88ef.png)
-
19690-69-8
3-bromo-[2,2’]bithiophenyl

-
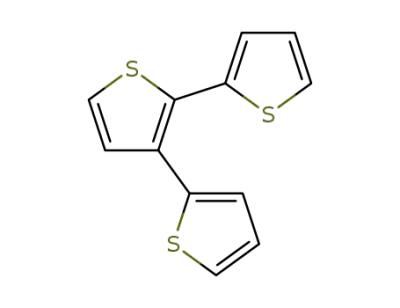
-
105124-96-7
2,2':3',2-terthiophene
| Conditions | Yield |
|---|---|
|
(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride;
In
diethyl ether;
at 0 ℃;
for 3h;
|
7% 82% |
492-97-7 Upstream products
-
188290-36-0

thiophene
-
30930-49-5
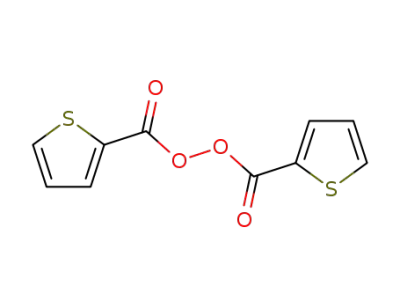
bis(2-thiophenecarbonyl) peroxide
-
1003-09-4
2-bromothiophene
-
3437-95-4
2-Iodothiophene
492-97-7 Downstream products
-
205643-77-2
2-([2,2']bithienyl-5-carbonyl)-benzoic acid
-
18494-73-0
5,5'-di(1-oxoethyl)-2,2'-bithiophene
-
3779-27-9
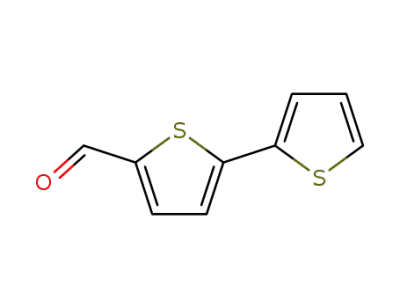
2,2'-bithiophene-5-carboxaldehyde
-
3339-80-8
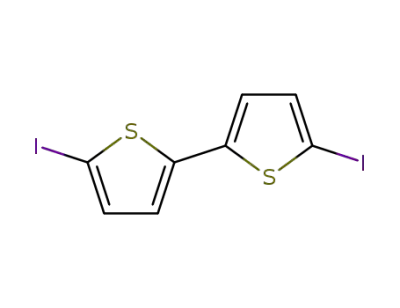
5,5'-diiodo-2,2-bithiophene
Relevant Products
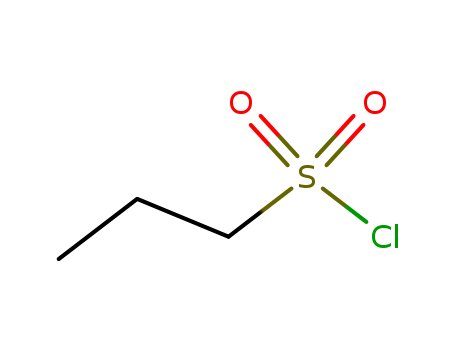
-
Propanesulphonyl chloride
CAS:10147-36-1
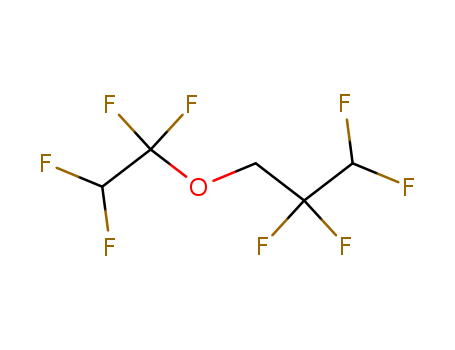
-
1,1,2, 2-tetrafluoroethyl -2,2,3, 3-tetrafluoropropyl ether
CAS:16627-68-2
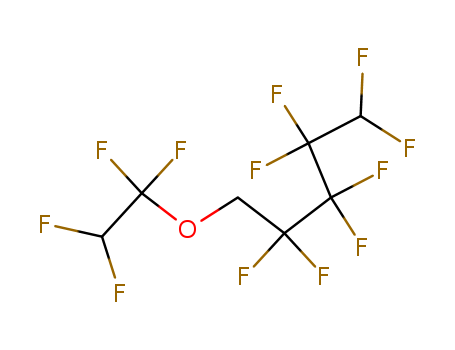
-
1H,1H,5H-Perfluoropentyl-1,1,2,2-tetrafluoroethylether
CAS:16627-71-7