116971-11-0
- Product Name:2,5-Dibromo-3-hexylthiophene
- Molecular Formula:C10H14Br2S
- Purity:99%
- Molecular Weight:326.095
Product Details;
CasNo: 116971-11-0
Molecular Formula: C10H14Br2S
Appearance: Light yellow liquid
factory and supplier 116971-11-0 2,5-Dibromo-3-hexylthiophene in stock
- Molecular Formula:C10H14Br2S
- Molecular Weight:326.095
- Appearance/Colour:Light yellow liquid
- Vapor Pressure:0.000726mmHg at 25°C
- Refractive Index:n20/D 1.557(lit.)
- Boiling Point:317.2 °C at 760 mmHg
- Flash Point:145.6 °C
- PSA:28.24000
- Density:1.551 g/cm3
- LogP:5.39590
2,5-Dibromo-3-hexylthiophene(Cas 116971-11-0) Usage
|
General Description |
2,5-Dibromo-3-hexylthiophene is a 2,5 coupled conductive polymer with conjugated polythiophene based system, which has a controllable band gap. |
InChI:InChI=1/C10H14Br2S/c1-2-3-4-5-6-8-7-9(11)13-10(8)12/h7H,2-6H2,1H3
116971-11-0 Relevant articles
Hierarchical helical assembly of conjugated poly(3-hexylthiophene)-block- poly(3-triethylene glycol thiophene) diblock copolymers
Lee, Eunji,Hammer, Brenton,Kim, Jung-Keun,Page, Zachariah,Emrick, Todd,Hayward, Ryan C.
, p. 10390 - 10393 (2011)
We report on the solution-state assembly...
Study of an efficient and selective bromination reaction of substituted thiophenes
Hoffmann, Kenneth J.,Carlsen, Per H. J.
, p. 1607 - 1610 (1999)
Bromination in concentrated solutions of...
Synthesis and photovoltaic properties of polythiophene incorporating with 3,4-difluorothiophene units
Huang, Linquan,Yang, Dong,Gao, Qiang,Liu, Yan,Lu, Shengmei,Zhang, Jian,Li, Can
, p. 1385 - 1390 (2013)
Two polythiophene derivatives using fluo...
Synthesis and Characterization of Diferrocenyl Conjugates: Varying π-Conjugated Bridging Ligands and its Consequence on Electrochemical Communication
Roy, Sourav Saha,Patra, Sanjib K.
, p. 2193 - 2201 (2019)
Organometallic wire-like complexes with ...
Preparation of near-infrared absorbing composites comprised of conjugated macroligands on the surface of PbS nanoparticles
Zhang, Jinming,Bahrig, Lydia,Puetz, Andreas,Kanelidis, Ioannis,Lenkeit, Daniel,Pelz, Simon,Hickey, Stephen G.,Klein, Michael F.G.,Colsmann, Alexander,Lemmer, Uli,Eychmüller, Alexander,Holder, Elisabeth
, p. 5525 - 5533 (2013)
We report a facile macroligand strategy ...
Synthesis, morphology, and field-effect transistor characteristics of new crystalline-crystalline diblock copolymers of poly(3-hexylthiophene-block-steryl acrylate)
Lin, Jung-Chuan,Lee, Wen-Ya,Kuo, Chi-Ching,Li, Chaoxu,Mezzenga, Raffaele,Chen, Wen-Chang
, p. 686 - 695 (2012)
We report the synthesis, morphology, and...
Controlling phase separation and optical properties in conjugated polymers through selenophene-thiophene copolymerization
Hollinger, Jon,Jahnke, Ashlee A.,Coombs, Neil,Seferos, Dwight S.
, p. 8546 - 8547 (2010)
Selenophene-thiophene block copolymers w...
Diruthenium(ii)-capped oligothienylethynyl bridged highly soluble organometallic wires exhibiting long-range electronic coupling
Saha Roy, Sourav,Sil, Amit,Giri, Dipanjan,Roy Chowdhury, Sabyasachi,Mishra, Sabyashachi,Patra, Sanjib K.
, p. 14304 - 14317 (2018)
Organometallic molecular wires with π-co...
Copolymerization of Polythiophene and Sulfur to Improve the Electrochemical Performance in Lithium-Sulfur Batteries
Oschmann, Bernd,Park, Jungjin,Kim, Chunjoong,Char, Kookheon,Sung, Yung-Eun,Zentel, Rudolf
, p. 7011 - 7017 (2015)
We first report on the copolymerization ...
An efficient and reliable procedure for the preparation of highly reactive Rieke zinc
Kudret, Suleyman,D'Haen, Jan,Lutsen, Laurence,Vanderzande, Dirk,Maes, Wouter
, p. 569 - 575 (2013)
Rieke zinc has a wide potential for appl...
Cross-linked conjugated polymer fibrils: Robust nanowires from functional polythiophene diblock copolymers
Hammer, Brenton A. G.,Bokel, Felicia A.,Hayward, Ryan C.,Emrick, Todd
, p. 4250 - 4256 (2011)
A series of poly(3-hexyl thiophene) (P3H...
Approaching the Integer-Charge Transfer Regime in Molecularly Doped Oligothiophenes by Efficient Decarboxylative Cross-Coupling
Forgione, Pat,Hase, Hannes,Liu, Jiang Tian,Salzmann, Ingo,Taylor, Sarah
supporting information, p. 7146 - 7153 (2020/03/23)
A library of symmetrical linear oligothi...
1,2,3-Triazolyl functionalized thiophene, carbazole and fluorene based A-: Alt -B type π-conjugated copolymers for the sensitive and selective detection of aqueous and vapor phase nitroaromatics (NACs)
Giri, Dipanjan,Patra, Sanjib K.
supporting information, p. 14469 - 14480 (2020/11/09)
A series of highly emissive π-conjugated...
116971-11-0 Process route
-

-
1693-86-3
3-hexylthiophene

-
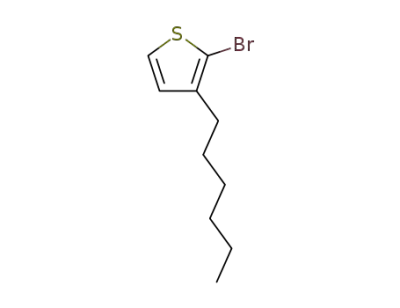
-
69249-61-2,125321-66-6
3-hexyl-2-bromothiophene

-
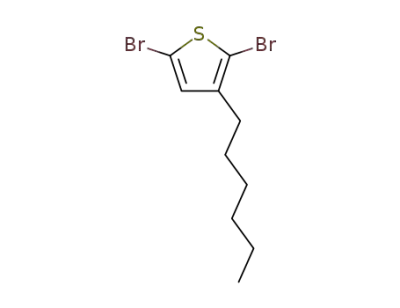
-
116971-11-0
2,5-dibromo-3-hexylthiophene
| Conditions | Yield |
|---|---|
|
With
N-Bromosuccinimide;
In
acetic acid;
for 0.833333h;
Yields of byproduct given;
|
91.7% |
-

-
1693-86-3
3-hexylthiophene

-

-
116971-11-0
2,5-dibromo-3-hexylthiophene
| Conditions | Yield |
|---|---|
|
With
N-Bromosuccinimide;
In
chloroform;
at 0 ℃;
|
99% |
|
With
N-Bromosuccinimide;
In
chloroform; N,N-dimethyl-formamide;
at 60 ℃;
|
98% |
|
With
hydrogen bromide; dihydrogen peroxide;
In
water;
at -5 - 20 ℃;
for 23h;
Product distribution / selectivity;
|
97% |
|
With
N-Bromosuccinimide;
In
chloroform; acetic acid;
for 0.5h;
Heating;
|
95% |
|
With
methanol; tetrabutylammomium bromide; bromine;
In
dichloromethane;
at 20 ℃;
|
95% |
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 25 ℃;
for 12h;
Inert atmosphere;
Schlenk technique;
|
92% |
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 20 ℃;
for 4.16667h;
Inert atmosphere;
|
88% |
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 25 ℃;
for 12h;
|
88% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 0 - 20 ℃;
Darkness;
|
81% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 0 - 20 ℃;
Darkness;
|
81% |
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 0 ℃;
for 2h;
Inert atmosphere;
|
80% |
|
With
N-Bromosuccinimide;
at 20 ℃;
Cooling with ice;
|
80% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 0 - 22.5 ℃;
for 16h;
Inert atmosphere;
|
80% |
|
With
N-Bromosuccinimide;
In
chloroform; N,N-dimethyl-formamide;
at 60 ℃;
Inert atmosphere;
|
71% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at -20 - 20 ℃;
for 5h;
|
70% |
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 0 - 20 ℃;
|
69% |
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 0 ℃;
Darkness;
|
64% |
|
With
bromine;
In
chloroform;
Ambient temperature;
|
|
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 20 ℃;
|
|
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 0 - 20 ℃;
Darkness;
Inert atmosphere;
|
|
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
|
|
|
With
N-Bromosuccinimide;
|
|
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 20 ℃;
for 4h;
Inert atmosphere;
|
8.5 g |
|
3-hexylthiophene;
With
N-Bromosuccinimide;
In
tetrahydrofuran;
|
|
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 0 - 5 ℃;
for 1h;
|
|
|
With
N-Bromosuccinimide;
In
dichloromethane;
Inert atmosphere;
|
|
|
With
N-Bromosuccinimide;
In
tetrahydrofuran; chloroform;
at 20 ℃;
Cooling with ice;
Inert atmosphere;
|
|
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
Inert atmosphere;
|
|
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 28 ℃;
for 12h;
|
116971-11-0 Upstream products
-
1693-86-3

3-hexylthiophene
-
69249-61-2

3-hexyl-2-bromothiophene
-
3761-92-0
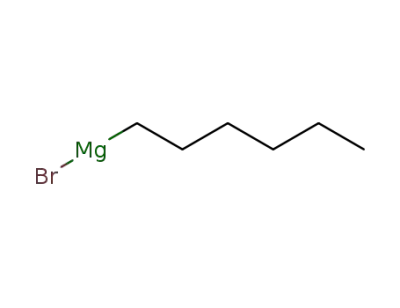
n-hexylmagnesium bromide
-
111-25-1
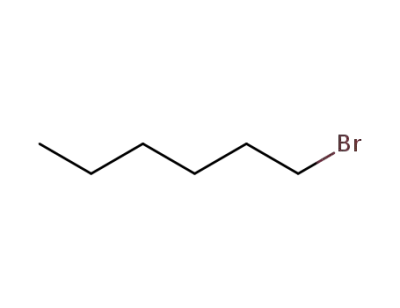
1-bromo-hexane
116971-11-0 Downstream products
-
492-97-7
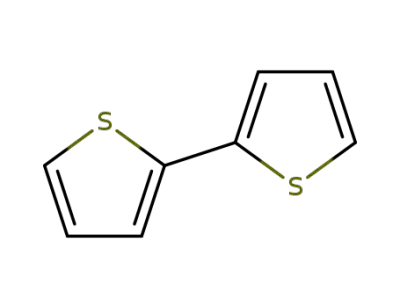
2,2'-Bithiophene
-
105124-96-7
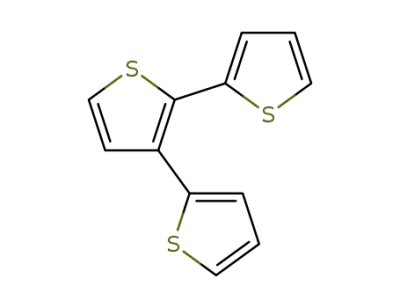
2,2':3',2-terthiophene
-
134628-84-5
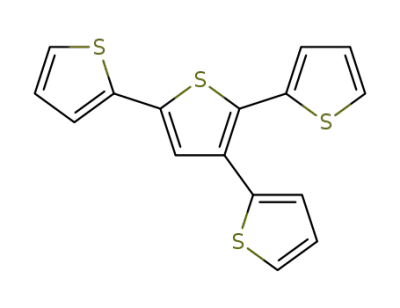
5'-(2-thienyl)-2,2':3',2-terthiophene
-
173448-32-3
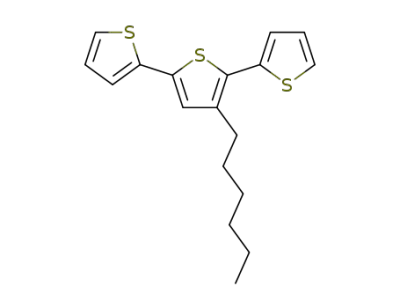
3'-hexyl-2,2':5',2-terthiophene
Relevant Products
-
Methyl 2-bromothiophene-3-carboxylate
CAS:76360-43-5
-
1-Ethyl-1-MethylpyrrolidiniuM broMide
CAS:69227-51-6
-
4-Chloro-2-fluoroaniline
CAS:57946-56-2