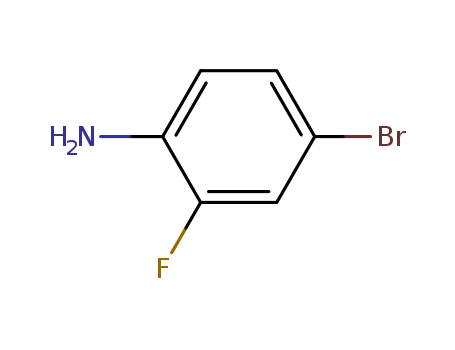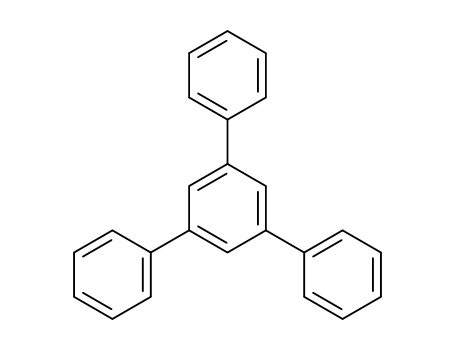
612-71-5
- Product Name:1,3,5-Triphenylbenzene
- Molecular Formula:C24H18
- Purity:99%
- Molecular Weight:306.407
Product Details;
CasNo: 612-71-5
Molecular Formula: C24H18
Appearance: LIGHT BROWN /CRYSTALLINE POWDER
factory and supplier 612-71-5 1,3,5-Triphenylbenzene in stock
- Molecular Formula:C24H18
- Molecular Weight:306.407
- Appearance/Colour:LIGHT BROWN /CRYSTALLINE POWDER
- Vapor Pressure:3.31E-08mmHg at 25°C
- Melting Point:172-174 °C(lit.)
- Refractive Index:1.4800 (estimate)
- Boiling Point:460 °C(lit.)
- Flash Point:238 ºC
- PSA:0.00000
- Density:1.074 g/cm3
- LogP:6.68760
1,3,5-Triphenylbenzene(Cas 612-71-5) Usage
|
Purification Methods |
Purify it by chromatography on alumina using *benzene or pet ether as eluents. Crystallise the triphenylbenzene from EtOH (m 174o). [Beilstein 5 H 737, 5 I 370, 5 II 670, 5 III 2563, 5 IV 2732.] |
InChI:InChI=1/C24H18/c1-4-10-19(11-5-1)22-16-23(20-12-6-2-7-13-20)18-24(17-22)21-14-8-3-9-15-21/h1-18H
612-71-5 Relevant articles
Efficient conversion of acetophenones into 1,3,5-triarylbenzenes catalyzed by bismuth(III) trifluoromethanesulfonate tetrahydrate
Ono, Fumiaki,Ishikura, Yuichi,Tada, Yuusuke,Endo, Masato,Sato, Tsuneo
, p. 2365 - 2367 (2008)
Bismuth(III) trifluoromethanesulfonate t...
Highly efficient and novel method for synthesis of 1,3,5-triarylbenzenes from acetophenones
Phatangare, Kiran,Padalkar, Vikas,Mhatre, Devidas,Patil, Ketan,Chaskar, Atul
, p. 4117 - 4121 (2009)
Heteropolyacid-phosphomolybdic acid has ...
Thionyl chloride-catalyzed preparation of microporous organic polymers through aldol condensation
Zhao, Yan-Chao,Zhou, Ding,Chen, Qi,Zhang, Xin-Jian,Bian, Ning,Qi, Ai-Di,Han, Bao-Hang
, p. 6382 - 6388 (2011)
We demonstrated the synthesis of five ki...
1,3,5-triphenylbenzene as unexpected by-product in the synthesis of 2,5-diphenylpyrrole from acetophenone oxime and phenylacetylene
Shmidt,Zorina,Mikhaleva,Ushakov,Skital'Tseva,Trofimov
, p. 457 - 458 (2010)
-
Efficient one-step synthesis of C 3-symmetrical benzenoid compounds mediated by SOCl 2/EtOH
Zhao, Sanhu,Kang, Lina,Ge, Haixia,Yang, Feifei,Wang, Chenlu,Li, Chang,Wang, Qiang,Zhao, Minggen
, p. 3569 - 3578 (2012)
An efficient one-step synthesis of branc...
Independent electrocyclization and oxidative chain cleavage along the backbone of cis-poly(phenylacetylene)
Percec, Virgil,Rudick, Jonathan G.
, p. 7241 - 7250 (2005)
cis-Poly(phenylacetylene) (PPA) and cis-...
TiCl3(OTf) catalyses the efficient conversion of acetophenones to 1,3,5-triaryl benzenes
Iranpoor,Zeynizaded
, p. 1079 - 1080 (1998)
The quantitative conversion of different...
Simple and convenient synthesis of 1,3,5-triarylbenzenes from ketones
Hu, Anan,Zhang, Anjiang,Ding, Lisheng,Lei, Xinxiang,Zhang, Lixue
, p. 720 - 721 (2007)
A new and efficient synthesis of a serie...
Silver(I) Interactions with Ketones. Site of Complexation with Acetophenones and Effectiveness as a Lewis Acid Catalyst
Crist, DeLanson R.,Hsieh, Zon-Hong,Quicksall, Carl O.,Sun, Michael K.
, p. 2478 - 2483 (1984)
Aromatic ketones present three possible ...
OLIGOMERISATION OF PHENYL ACETYLENE OVER TITANIUM OXIDE SUPPORTED ON SILICA - ALUMINA CATALYST
Kumar, V. G.,Shoba, T. S.,Rao, K. V. C.
, p. 6245 - 6248 (1985)
Titanium oxide on silica-alumina support...
2-cyano-iso-propyl radical addition to alkynes
Montevecchi, Pier Carlo,Navacchia, Maria Luisa,Spagnolo, Piero
, p. 7929 - 7936 (1997)
The thermolysis of azobisisobutyronitril...
Fabrication of palladium nanoparticles as effective catalysts by using supramolecular gels
Zhang, Wei,Xie, Zhi-Gang
, p. 77 - 80 (2016)
Two-component supramolecular gels were m...
Chiral diphosphine derivatives of alkylidyne tricobalt carbonyl clusters - A comparative study of different cobalt carbonyl (pre)catalysts for (asymmetric) intermolecular Pauson-Khand reactions
Mottalib, M. Abdul,Haukka, Matti,Nordlander, Ebbe
, p. 275 - 282 (2016)
Reaction of the tricobalt carbyne cluste...
SRN1 and stille reactions: A new synthetic strategy
Corsico, Eduardo F.,Rossi, Roberto A.
, p. 431 - 432 (2000)
The photostimulated reaction of Me3Sn- i...
Catalytic three-component coupling of alkynes, imines, and organoboron reagents
Patel, Sejal J.,Jamison, Timothy F.
, p. 1364 - 1367 (2003)
An unusual degree of functional group co...
A facile synthesis of 1,3,5-triaryl benzenes from acetophenone diethyl ketals in the presence of acetyl chloride and SmCl3
Cheng, Ke-Jun,Ding, Zong-Biao,Wu, Shi-Hui
, p. 11 - 15 (1997)
Samarium trichloride, SmCl3, has been fo...
Effect of phosphonium ionic liquid/Pd ratio on the catalytic activity of palladium nanoparticles in Suzuki cross-coupling reaction
Arkhipova, D.,Ermolaev, V.,Gaynanova, G.,Hey-Hawkins, E.,Miluykov, V.,Oeckler, O.,Wagner, G.,Zakharova, L.
, (2020)
Phosphonium salts were synthesized and u...
Suzuki cross-coupling of hexachlorobenzene promoted by the Buchwald ligands
Burukin, A. S.,Vasil’ev, A. A.,Zhdankina, G. M.,Zlotin, S. G.
, p. 169 - 172 (2022/02/17)
The study of cross-coupling between hexa...
Variation on the π-Acceptor Ligand within a RhI?N-Heterocyclic Carbene Framework: Divergent Catalytic Outcomes for Phenylacetylene-Methanol Transformations
Galiana-Cameo, María,Passarelli, Vincenzo,Pérez-Torrente, Jesús J.,Di Giuseppe, Andrea,Castarlenas, Ricardo
, p. 2947 - 2957 (2021/07/16)
A series of neutral and cationic rhodium...
Hydrogen-Bonding Controlled Nickel-Catalyzed Regioselective Cyclotrimerization of Terminal Alkynes
Yang, Kai,Wang, Pengfei,Sun, Ze-Ying,Guo, Minjie,Zhao, Wentao,Tang, Xiangyang,Wang, Guangwei
supporting information, p. 3933 - 3938 (2021/05/26)
Herein we report a hydrogen-bonding cont...
Iron-catalyzed trimerization of terminal alkynes enabled by pyrimidinediimine ligands: A regioselective method for the synthesis of 1,3,5-substituted arenes
Doll, Julianna S.,Eichelmann, Robert,Hertwig, Leif E.,Bender, Thilo,Kohler, Vincenz J.,Bill, Eckhard,Wadepohl, Hubert,Ro?ca, Drago?-Adrian
, p. 5593 - 5600 (2021/05/31)
The development of pyrimidine-based anal...
612-71-5 Process route
-
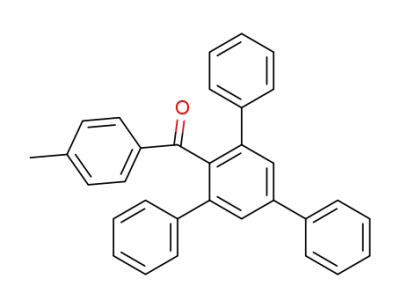
-
130894-36-9
2,4,6-Triphenyl,4'-methylbenzophenone

-
-
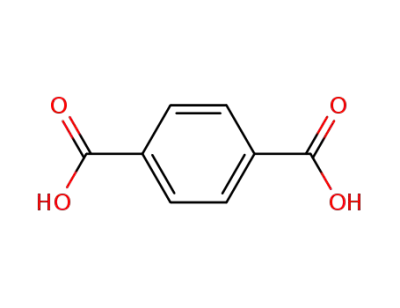
100-21-0
terephthalic acid

-
-
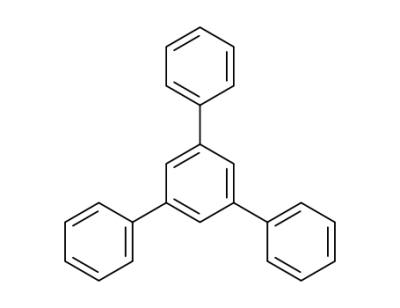
612-71-5
1,3,5-triphenylbenzene

-

-
99-94-5
p-Toluic acid
| Conditions | Yield |
|---|---|
|
at 275 - 285 ℃;
|
-
-
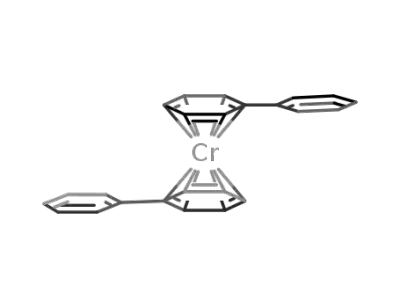
12099-15-9
bis(η6-diphenyl)chromium(0)

-
-
12087-58-0
bis(toluene)chromium(0)

-
-
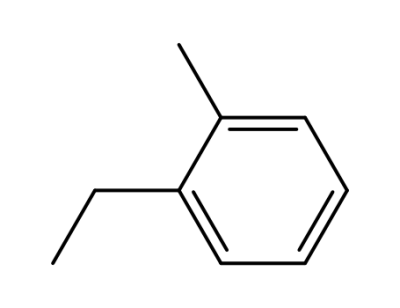
32875-80-2
C6H6CrC6H5C2H5

-
-
611-14-3
2-Ethyltoluene

-
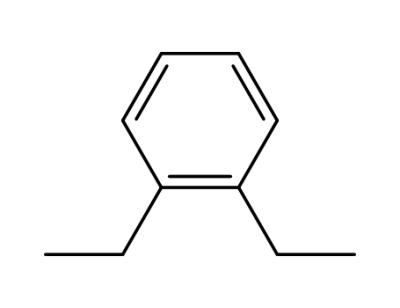
-
253185-02-3,135-01-3
ortho-diethylbenzene

-

-
612-71-5
1,3,5-triphenylbenzene
| Conditions | Yield |
|---|---|
|
Friedel-Crafts synthesis; chromy.;
|
|
|
Friedel-Crafts synthesis; chromy.;
|
612-71-5 Upstream products
-
493-72-1
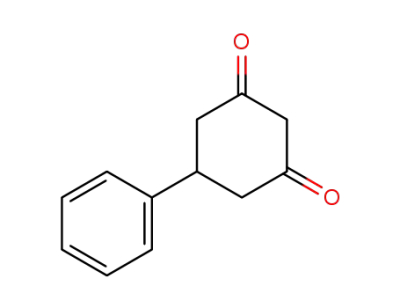
5-phenyl-cyclohexane-1,3-dione
-
60-29-7
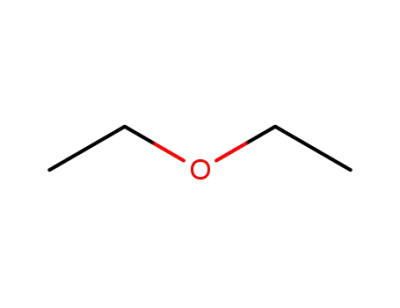
diethyl ether
-
512-85-6
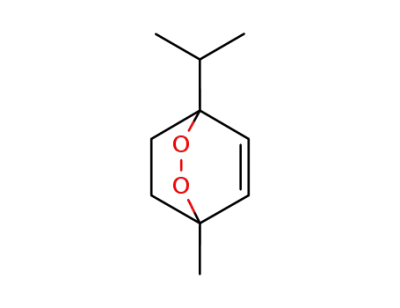
ascaridole
-
618-34-8
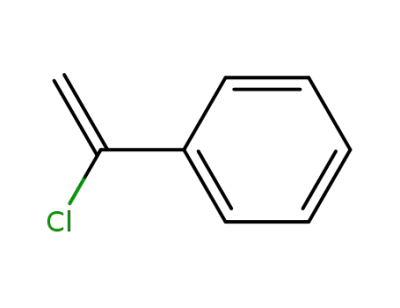
1-chlorostyrene
612-71-5 Downstream products
-
10368-73-7
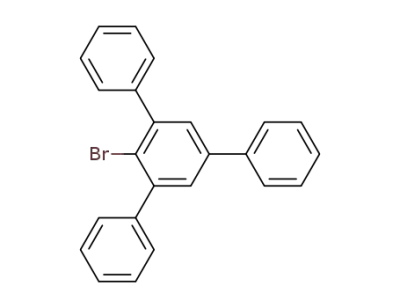
2-bromo-1,3,5-triphenylbenzene
-
6297-08-1
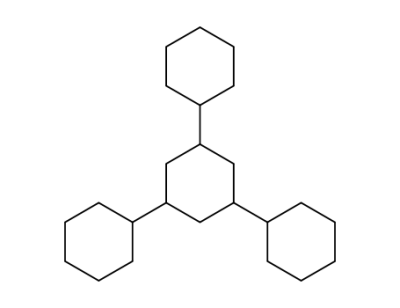
1,3,5-tricyclohexyl-cyclohexane
-
7371-00-8
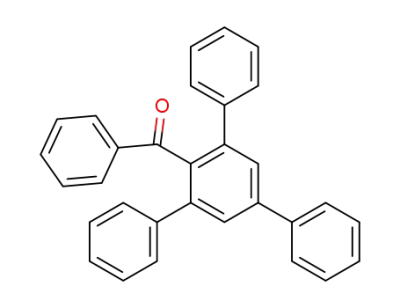
2,4,6-Triphenyl-benzophenon
-
130894-36-9

2,4,6-Triphenyl,4'-methylbenzophenone
Relevant Products
-
DIBASIC ESTER
CAS:95481-62-2
-
4-Bromo-2-fluoroaniline
CAS:367-24-8
-
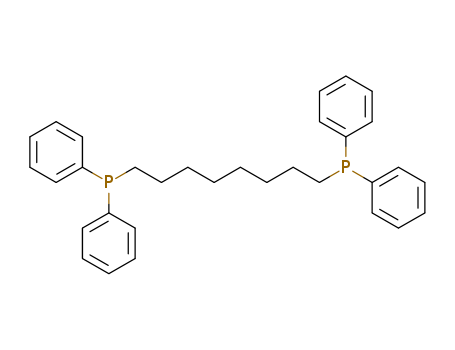
1,8-Bis(diphenylphosphino)octane
CAS:41625-30-3