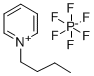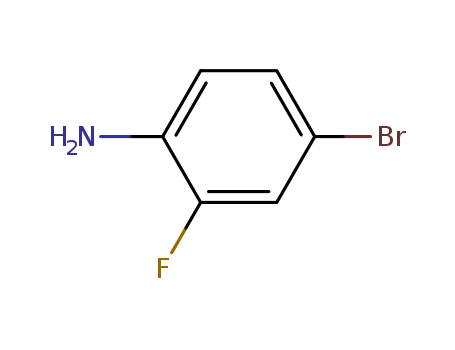
678-39-7
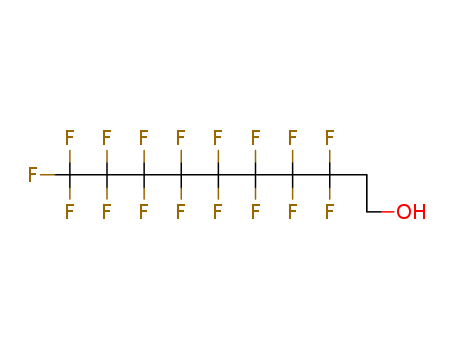
- Product Name:1H,1H,2H,2H-Perfluoro-1-decanol
- Molecular Formula:C10H5F17O
- Purity:99%
- Molecular Weight:464.122
Product Details;
CasNo: 678-39-7
Molecular Formula: C10H5F17O
factory and supplier 678-39-7 1H,1H,2H,2H-Perfluoro-1-decanol in stock
- Molecular Formula:C10H5F17O
- Molecular Weight:464.122
- Vapor Pressure:0.000484mmHg at 25°C
- Melting Point:50 °C
- Refractive Index:1.33
- Boiling Point:187.9 °C at 760 mmHg
- PKA:14.28±0.10(Predicted)
- Flash Point:67.5 °C
- PSA:20.23000
- Density:1.633g/cm3
- LogP:5.37820
1H,1H,2H,2H-Perfluoro-1-decanol(Cas 678-39-7) Usage
|
Definition |
ChEBI: A fluorotelomer alcohol that is ethanol substituted at position 2 by a perfluorooctyl group. |
|
General Description |
1H,1H,2H,2H-Perfluoro-1-decanol (8:2 FTOH) is an 8:2 fluorotelomer alcohol. The quantitative analysis of 8:2 FTOH in soil can be done using GC/MS with instrument detection limit (IDL) of 10fg/L. Studies indicate that hydroxyl radical causes indirect photodegradation of 8:2 FTOH in aqueous media. |
InChI:InChI=1/C8H2F12O4/c9-3(10,1(21)22)5(13,14)7(17,18)8(19,20)6(15,16)4(11,12)2(23)24/h(H,21,22)(H,23,24)
678-39-7 Relevant articles
Electrochemical Oxidation of Polyfluoroalkyl Iodides: Direct Anodic Transformation of C8F17CH2CH2I to Amides, Esters, and Ethers
Becker, James Y.,Smart, Bruce E.,Fukunaga, Tadamichi
, p. 5714 - 5720 (1988)
The cyclic voltammetry of polyfluoroalky...
Method for preparing perfluoroalkyl ethanol
-
Paragraph 0026-0032, (2017/11/29)
The invention discloses a method for pre...
Method for preparing perfluoroalkyl alcohol from perfluoroalkyl ethylene
-
Paragraph 0034; 0036, (2017/04/03)
The invention discloses a method for pre...
Unprecedented iron-catalyzed ester hydrogenation. Mild, selective, and efficient hydrogenation of trifluoroacetic esters to alcohols catalyzed by an iron pincer complex
Zell, Thomas,Ben-David, Yehoshoa,Milstein, David
supporting information, p. 4685 - 4689 (2014/05/20)
The synthetically important, environment...
Synthesis of 2-(perfluoroalkyl)ethyl potassium sulfates based on perfluorinated Grignard reagents
Paterová, Jana,Skalicky, Martin,Rybá?ková, Markéta,Kví?alová, Magdalena,Cva?ka, Josef,Kví?ala, Jaroslav
experimental part, p. 1338 - 1343 (2011/02/22)
The first example of nucleophilic substi...
678-39-7 Process route
-
-
116-14-3,82785-14-6
polytetrafluoroethylene

-
-
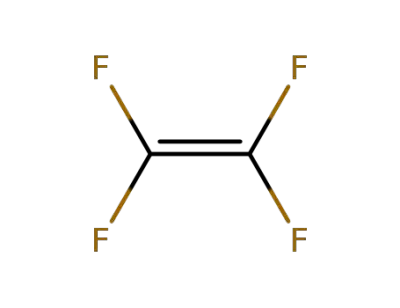
74-85-1
ethene

-
-
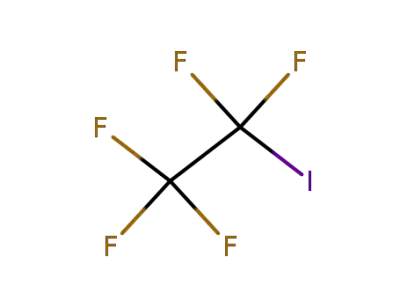
354-64-3
Pentafluoroethyl iodide

-
-
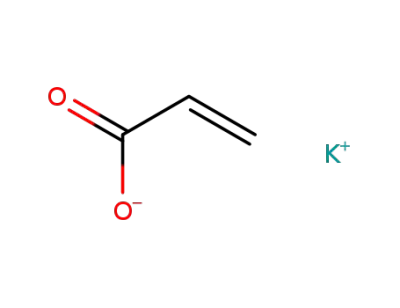
10192-85-5
potassium acrylate

-
-
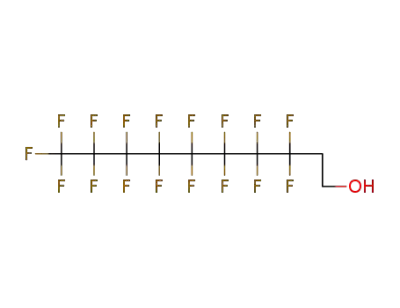
865-86-1
2-(perfluorodecyl)ethanol

-
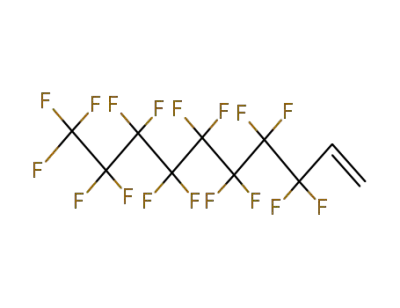
-
21652-58-4
1H,1H,2H-perfluorodecene

-
-
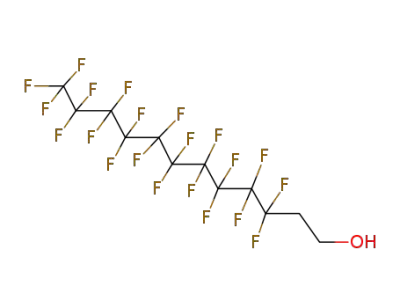
678-39-7
3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,10-heptadecafluoro-1-decanol

-
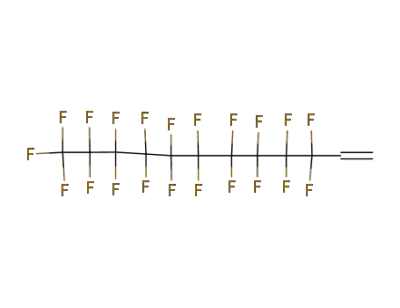
-
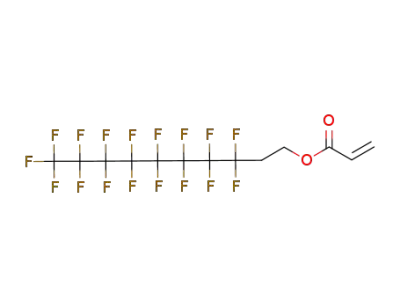
30389-25-4
3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,12,12,12-henicosafluorododec-1-ene

-
-
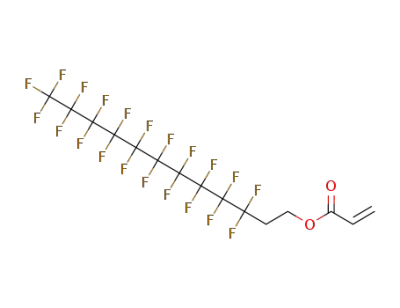
27905-45-9
1H,1H,2H,2H-heptadecafluorodecyl acrylate

-
-
17741-60-5
1,1,2,2-Tetrahydroperfluorododecyl acrylate
| Conditions | Yield |
|---|---|
|
polytetrafluoroethylene; Pentafluoroethyl iodide;
copper catalyst;
at 80 ℃;
under 6000.6 Torr;
ethene;
copper catalyst;
at 100 ℃;
for 3h;
under 2250.23 Torr;
potassium acrylate;
With
4-methoxy-phenol; hydroquinone;
In
tert-butyl alcohol;
at 180 - 190 ℃;
for 6h;
Product distribution / selectivity;
|
-
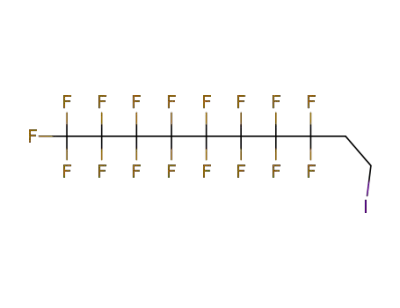
-
2043-53-0,71215-70-8
2-(n-perfluorooctyl)ethyl iodide

-

-
10192-85-5
potassium acrylate

-
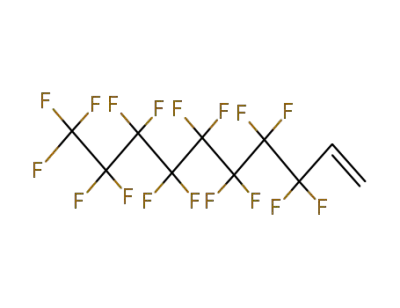
-
21652-58-4
1H,1H,2H-perfluorodecene

-

-
678-39-7
3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,10-heptadecafluoro-1-decanol

-

-
27905-45-9
1H,1H,2H,2H-heptadecafluorodecyl acrylate
| Conditions | Yield |
|---|---|
|
With
4-methoxy-phenol; hydroquinone;
In
tert-butyl alcohol;
at 180 - 190 ℃;
for 6h;
Product distribution / selectivity;
|
678-39-7 Upstream products
-
74-85-1
ethene
-
77758-89-5
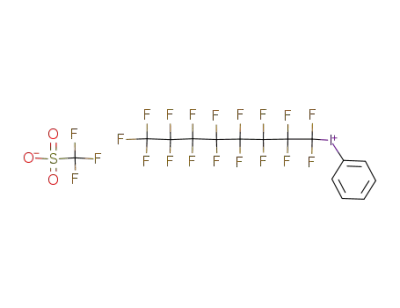
phenyl(perfluorooctyl)iodonium triflate
-
2043-53-0
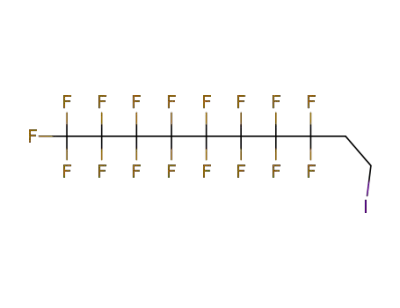
2-(n-perfluorooctyl)ethyl iodide
-
117068-31-2
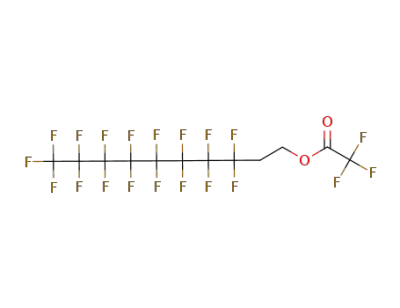
(3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,10-heptadecafluoro-n-decyl)trifluoroacetate
678-39-7 Downstream products
-
1996-88-9
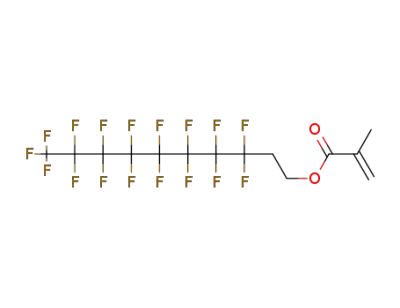
3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,10-heptadecafluorodecyl methacrylate
-
27854-31-5
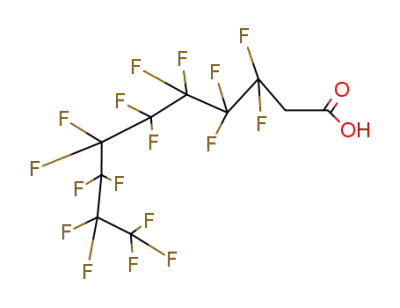
acide 2-perfluorooctylethanoieque
-
201593-46-6
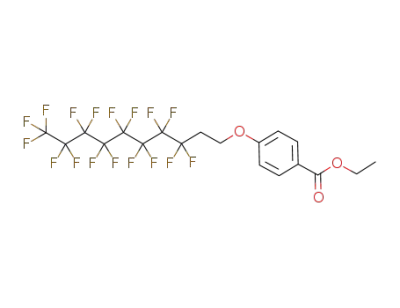
ethyl 4-<2-(perfluorooctyl)ethoxy>benzoate
-
1829-26-1
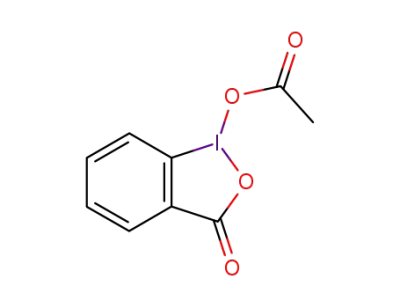
1-acetoxy-1,2-benziodoxol-3-one
Relevant Products
-
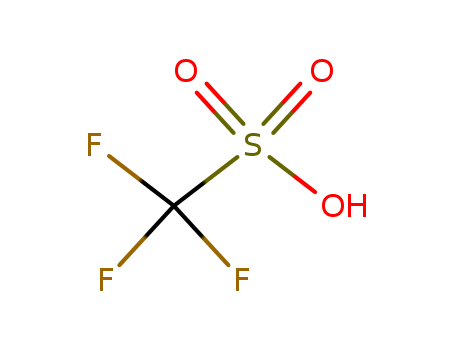
Trifluoromethanesulfonic acid
CAS:1493-13-6
-
N-Butylpyridinium hexafluorophosphate
CAS:186088-50-6
-
4-Bromo-2-fluoroaniline
CAS:367-24-8