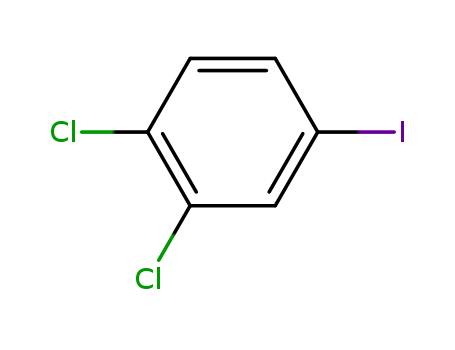
20555-91-3
- Product Name:3,4-Dichloroiodobenzene
- Molecular Formula:C6H3Cl2I
- Purity:99%
- Molecular Weight:272.9
Product Details;
CasNo: 20555-91-3
Molecular Formula: C6H3Cl2I
Appearance: beige low melting solid
factory and supplier 20555-91-3 3,4-Dichloroiodobenzene in stock
- Molecular Formula:C6H3Cl2I
- Molecular Weight:272.9
- Appearance/Colour:beige low melting solid
- Vapor Pressure:0.0214mmHg at 25°C
- Melting Point:27-29 °C(lit.)
- Refractive Index:1.6480
- Boiling Point:259 °C at 760 mmHg
- Flash Point:110.5 °C
- PSA:0.00000
- Density:2.015 g/cm3
- LogP:3.59800
3,4-Dichloroiodobenzene(Cas 20555-91-3) Usage
|
General Description |
3,4-Dichloroiodobenzene undergoes Stille coupling reaction with vinyltributyltin in the presence of Pd(0)precursors to furnish styrene derivative. |
InChI:InChI=1/C6H3Cl2I/c7-5-2-1-4(9)3-6(5)8/h1-3H
20555-91-3 Relevant articles
Rapid and efficient copper-catalyzed finkelstein reaction of (hetero)aromatics under continuous-flow conditions
Chen, Mao,Ichikawa, Saki,Buchwald, Stephen L.
supporting information, p. 263 - 266 (2015/02/05)
A general, rapid, and efficient method f...
Sterically controlled iodination of arenes via iridium-catalyzed C-H borylation
Partridge, Benjamin M.,Hartwig, John F.
, p. 140 - 143 (2013/03/28)
A mild method to prepare aryl and hetero...
TRICYCLIC COMPOUNDS AS mPGES-1 INHIBITORS
-
Page/Page column 44-45, (2012/09/10)
The present invention relates to tricycl...
Aryl sulfonamide and sulfonyl compounds as modulators of PPAR and methods of treating metabolic disorders
-
Page/Page column 59, (2010/02/14)
Aryl sulfonamide and sulfonyl compounds ...
20555-91-3 Process route
-
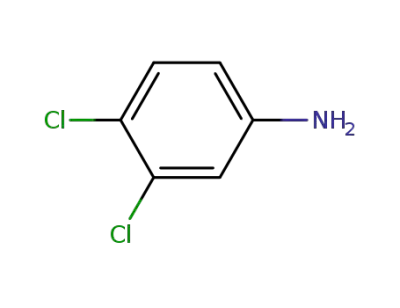
-
95-76-1
m,p-dichloroaniline

-
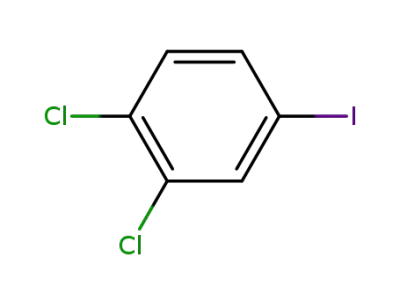
-
20555-91-3
3,4-dichloroiodobenzene
| Conditions | Yield |
|---|---|
|
m,p-dichloroaniline;
With
sulfuric acid;
In
water;
at 80 ℃;
for 0.166667h;
With
sodium nitrite;
In
water;
at 5 ℃;
for 1h;
With
potassium iodide;
In
water;
at 40 ℃;
for 1h;
|
79% |
|
Diazotization.Behandlung der Diazoniumsalz-Loesung mit KI;
|
|
|
Multistep reaction;
(i) NaNO2, aq. HCl, (ii) KI;
|
|
|
With
hydrogenchloride; potassium iodide; sodium nitrite;
Yield given. Multistep reaction;
1.)0-5 deg C; 2.)heated;
|
-
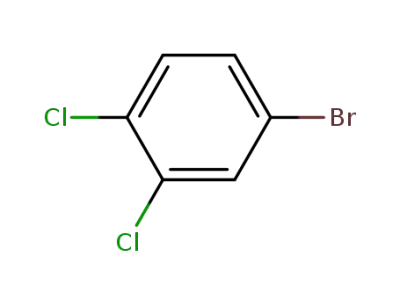
-
18282-59-2
4-bromo-1,2-dichlorobenzene

-
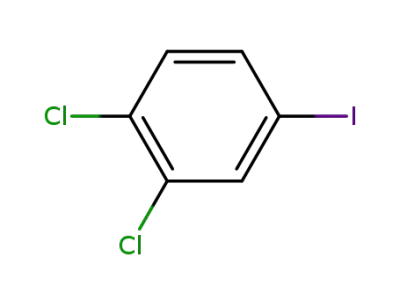
-
20555-91-3
3,4-dichloroiodobenzene
| Conditions | Yield |
|---|---|
|
With
copper(l) iodide; (R,R)-N,N'-dimethyl-1,2-diaminocyclohexane; sodium iodide;
In
1,4-dioxane; dichloromethane;
at 180 ℃;
for 0.5h;
under 9050.33 Torr;
Flow reactor;
Inert atmosphere;
|
87% |
20555-91-3 Upstream products
-
95-76-1

m,p-dichloroaniline
-
16156-46-0
3.4-Dichlor-benzoyliodid
-
3024-72-4
3,4-dichlorobenzoyl chloride
-
95-50-1
1,2-dichloro-benzene
20555-91-3 Downstream products
-
95-77-2
3,4-dichlorophenol
-
289039-26-5
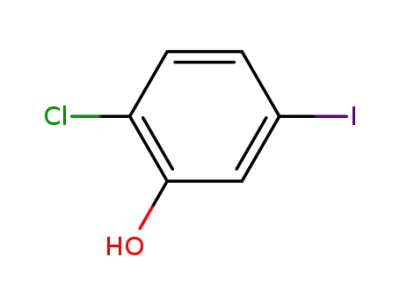
2-chloro-5-iodophenol
-
83665-60-5
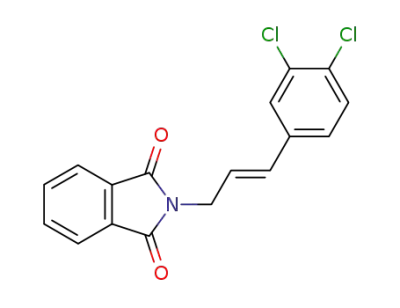
(E)-2-(3-(3,4-dichlorophenyl)allyl)isoindoline-1,3-dione
-
158723-71-8
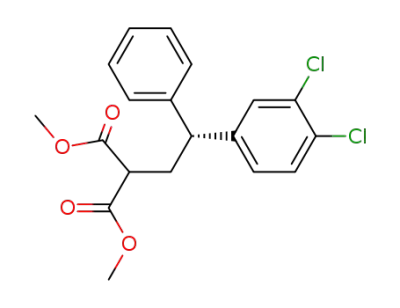
(R)-methyl 4-(3,4-dichlorophenyl)-4-phenyl-2-methoxycarbonylbutanoate
Relevant Products
-
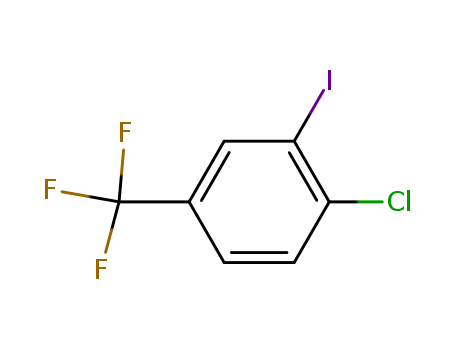
4-Chloro-3-iodobenzoTrifluoride
CAS:672-57-1
-
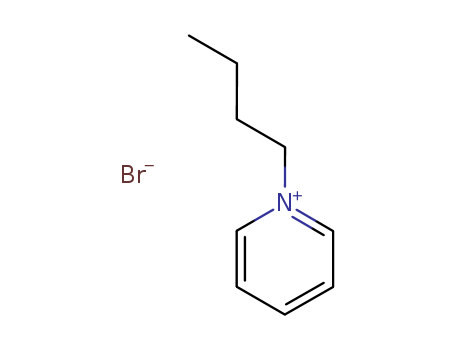
N-butyl pyridinium bromide
CAS:874-80-6