2035-75-8
- Product Name:Adipic Anhydride
- Molecular Formula:C6H8O3
- Purity:99%
- Molecular Weight:128.128
Product Details;
CasNo: 2035-75-8
Molecular Formula: C6H8O3
factory and supplier 2035-75-8 Adipic Anhydride in stock
- Molecular Formula:C6H8O3
- Molecular Weight:128.128
- Vapor Pressure:2.44mmHg at 25°C
- Melting Point:60-64 °C
- Refractive Index:1.456
- Boiling Point:262.1 °C at 760 mmHg
- Flash Point:123.9 °C
- PSA:43.37000
- Density:1.183 g/cm3
- LogP:0.63020
ADIPIC ANHYDRIDE(Cas 2035-75-8) Usage
InChI:InChI=1/C6H8O3/c1-2-3-4-5(7)9-6(4)8/h4H,2-3H2,1H3
2035-75-8 Relevant articles
-
Hill
, p. 4110,4113 (1930)
-
Hybrid inhibitors of phosphatidylinositol 3-kinase (PI3K) and the mammalian target of rapamycin (mTOR): Design, synthesis, and superior antitumor activity of novel wortmannin-rapamycin conjugates
Ayral-Kaloustian, Semiramis,Gu, Jianxin,Lucas, Judy,Cinque, Michael,Gaydos, Christine,Zask, Arie,Chaudhary, Inder,Wang, Jianyao,Di, Li,Young, Mairead,Ruppen, Mark,Mansour, Tarek S.,Gibbons, James J.,Yu, Ker
, p. 452 - 459 (2010)
Hyperactivation of the PI3K/AKT/mTOR sig...
Preparation method 6 -hydroxy caproic acid
-
Paragraph 0056-0060; 0093; 0095-0098; 0107; 0109-0112, (2021/11/10)
The invention provides a preparation met...
Unified Synthesis of Polycyclic Alkaloids by Complementary Carbonyl Activation**
Christmann, Mathias,He, Guoli,List, Benjamin
supporting information, p. 13591 - 13596 (2021/05/07)
A complementary dual carbonyl activation...
Evodiamine prodrug containing indole quinone unit as well as preparation method and application thereof
-
Paragraph 0026, (2021/10/30)
The invention relates to an evodiamine p...
Synthesis, Antiproliferative Activity, and Effect on Carcinoma A549 Cell Microtubules of New Tubuloclustin Analogs
Zefirov,Evteeva, Yu. A.,Fatkulin,Schulz,Kuznetsov,Zefirova
, p. 423 - 428 (2019/09/16)
Combretastatin analogs of the antitumor ...
2035-75-8 Process route
-
-
124-04-9
Adipic acid

-
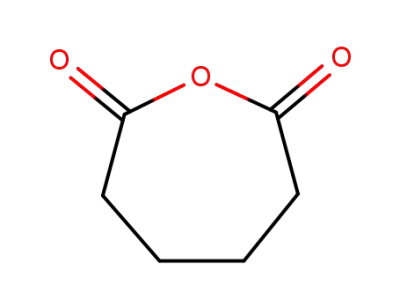
-
2035-75-8
adipic anhydride
| Conditions | Yield |
|---|---|
|
With
thionyl chloride; sodium carbonate;
In
1,4-dioxane; dichloromethane;
for 3h;
Heating;
|
84% |
|
Adipic acid;
With
acetic anhydride;
at 160 ℃;
for 4h;
zinc diacetate;
at 100 - 200 ℃;
|
82.5% |
|
Adipic acid;
With
acetic anhydride;
at 160 ℃;
for 4h;
Heating / reflux;
zinc diacetate;
at 100 - 200 ℃;
|
82.5% |
|
With
acetic anhydride;
for 12h;
Heating;
|
78% |
|
With
acetic anhydride;
for 4h;
Inert atmosphere;
Reflux;
|
77% |
|
With
trifluoroacetic anhydride;
In
ethyl acetate;
at 20 ℃;
for 24h;
|
68% |
|
at 180 ℃;
for 12h;
Temperature;
|
57.9% |
|
With
acetic anhydride;
for 4h;
Heating;
|
23% |
|
With
acetic anhydride;
for 1h;
Heating / reflux;
|
|
|
With
acetic anhydride;
at 90 ℃;
for 2h;
|
|
|
With
acetic anhydride;
for 1.5h;
Reflux;
|
|
|
With
acetic anhydride;
for 4h;
Heating / reflux;
|
|
|
With
acetic anhydride;
|
|
|
With
acetic anhydride;
Inert atmosphere;
Schlenk technique;
|
|
|
With
acetyl chloride;
for 1.5h;
Reflux;
|
-
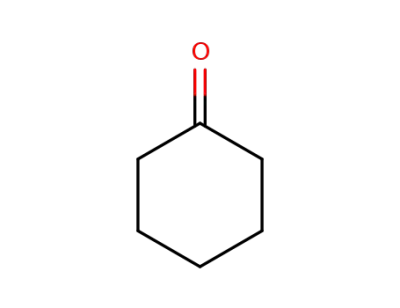
-
108-94-1,11119-77-0,9003-41-2,9075-99-4
cyclohexanone

-
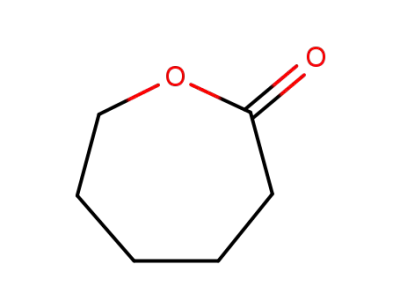
-
502-44-3,24980-41-4,80137-66-2
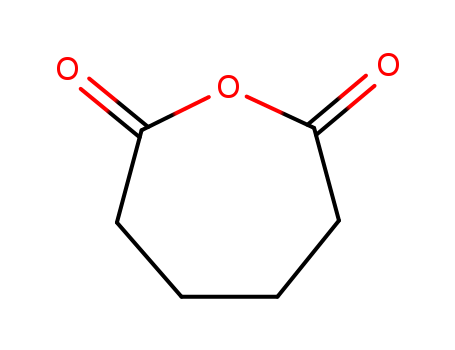
hexahydro-2H-oxepin-2-one

-

-
2035-75-8
adipic anhydride
| Conditions | Yield |
|---|---|
|
With
N-hydroxyphthalimide; ammonium cerium (IV) nitrate; oxygen;
In
acetonitrile;
at 45 ℃;
under 760.051 Torr;
|
2035-75-8 Upstream products
-
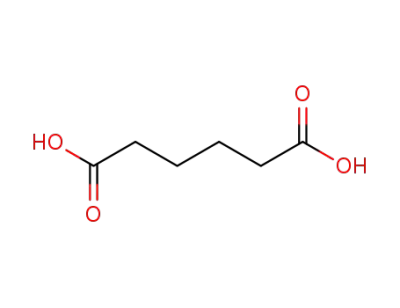
124-04-9

Adipic acid
-
108-24-7
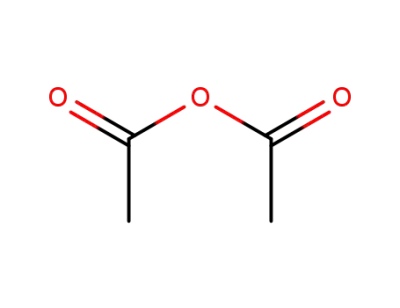
acetic anhydride
-
110-82-7
cyclohexane
-
108-94-1

cyclohexanone
2035-75-8 Downstream products
-
32564-25-3
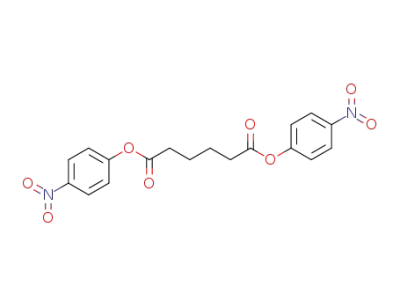
bis(4-nitrophenyl)adipate
-
73430-11-2
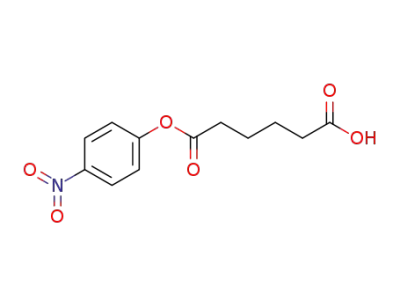
mono-p-nitrophenyl adipate
-
5538-18-1
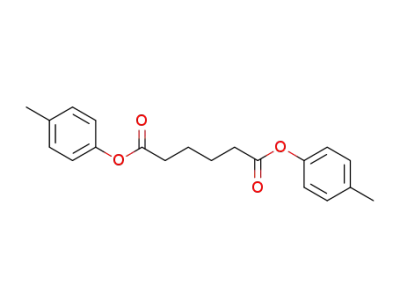
adipic acid di-p-tolyl ester
-
109261-98-5
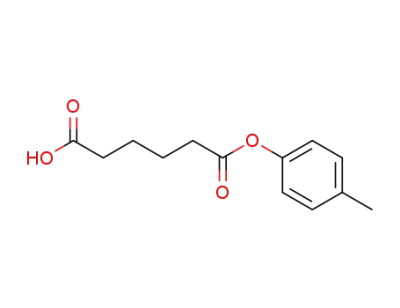
adipic acid mono-p-tolyl ester
Relevant Products
-
DIBASIC ESTER
CAS:95481-62-2
-
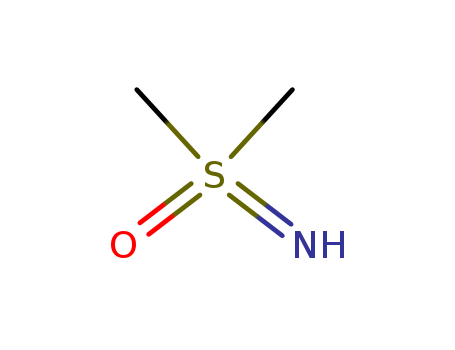
S,S-dimethyl sulfoximine
CAS:1520-31-6
-
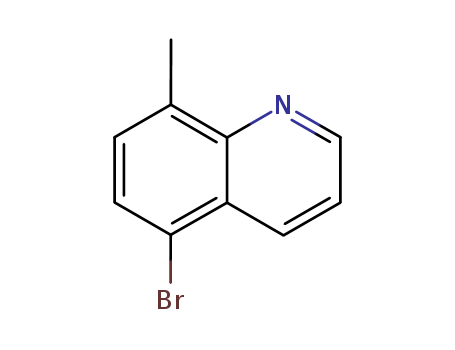
5-Bromo-8-methylquinoline
CAS:74316-55-5