2758-18-1
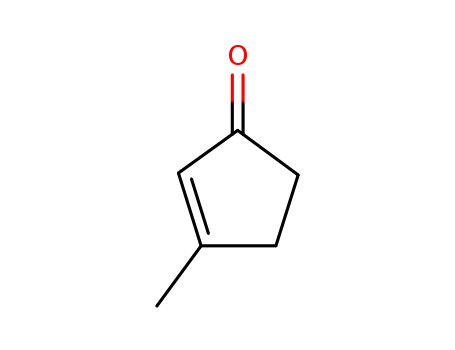
- Product Name:3-methylcyclopent-2-en-1-one
- Molecular Formula:C6H8O
- Purity:99%
- Molecular Weight:96.1289
Product Details;
CasNo: 2758-18-1
Molecular Formula: C6H8O
Appearance: clear light yellow to yellow-brownish liquid
factory and supplier 2758-18-1 3-methylcyclopent-2-en-1-one in stock
- Molecular Formula:C6H8O
- Molecular Weight:96.1289
- Appearance/Colour:clear light yellow to yellow-brownish liquid
- Vapor Pressure:2.74mmHg at 25°C
- Melting Point:3-5 °C(lit.)
- Refractive Index:n20/D 1.488(lit.)
- Boiling Point:157.5 °C at 760 mmHg
- Flash Point:53.1 °C
- PSA:17.07000
- Density:0.996 g/cm3
- LogP:1.29560
3-Methyl-2-cyclopenten-1-one(Cas 2758-18-1) Usage
|
Preparation |
By dehydrohalogenation of 2-chloro-1-methyl-cyclopentan-3-one. |
|
Synthesis Reference(s) |
Journal of the American Chemical Society, 101, p. 494, 1979 DOI: 10.1021/ja00496a044The Journal of Organic Chemistry, 55, p. 371, 1990 |
|
General Description |
3-Methyl-2-cyclopenten-1-one is a key intermediate in the synthesis of organolithium derivatives for natural product synthesis. It can be efficiently purified with high yield (94%) and serves as a precursor for brominated and acetalized derivatives, which are valuable in synthetic chemistry. The optimized procedures for its preparation and subsequent transformations emphasize high efficiency, environmental friendliness, and minimal waste generation. |
InChI:InChI=1/C6H8O/c1-5-2-3-6(7)4-5/h4H,2-3H2,1H3
2758-18-1 Relevant articles
Vapor-phase intramolecular aldol condensation of 2,5-hexanedione to 3-methylcyclopent-2-enone over ZrO2-supported Li2O catalyst
Sun, Daolai,Chiba, Shigenori,Yamada, Yasuhiro,Sato, Satoshi
, p. 105 - 108 (2017)
Vapor-phase intramolecular aldol condens...
Total synthesis of jiadifenolide
Paterson, Ian,Xuan, Mengyang,Dalby, Stephen M.
, p. 7286 - 7289 (2014)
As a potent neurotrophic agent, the sesq...
Synthesis of Bio-Based Methylcyclopentadiene from 2,5-Hexanedione: A Sustainable Route to High Energy Density Jet Fuels
Woodroffe, Josanne-Dee,Harvey, Benjamin G.
, p. 339 - 343 (2021)
The sustainable, bio-based, platform che...
-
Acheson,Robinson
, p. 1127,1131 (1952)
-
-
Mironov,V.A.,Akhrem,A.A.
, (1973)
-
Tantalum vs Niobium MCF nanocatalysts in the green synthesis of chromene derivatives
Smuszkiewicz, Agata,López-Sanz, Jesús,Sobczak, Izabela,Martín-Aranda, Rosa M.,Ziolek, Maria,Pérez-Mayoral, Elena
, p. 47 - 52 (2019)
TaMCF silicas modified with alkaline met...
Calcium and nitrogen species loaded into SBA-15-a promising catalyst tested in Knoevenagel condensation
Kryszak, Dorota,Stawicka, Katarzyna,Trejda, Maciej
, p. 9781 - 9794 (2020)
Mesoporous silica of the SBA-15 type was...
-
Mironov,V.A. et al.
, (1973)
-
Transformation of γ-ketoaldehyde acetals into 3-substituted-2-cyclopentenones via cyanophosphates under mild conditions
Yoneyama, Hiroki,Takatsuji, Kumi,Ito, Aiko,Usami, Yoshihide,Harusawa, Shinya
, (2021/02/06)
The reaction of cyanophosphates, which a...
Tris(2-aminoethyl)amine/metal oxides hybrid materials—preparation, characterization and catalytic application
Stawicka, Katarzyna,Ziolek, Maria
, (2020/10/29)
Three different metal oxides (basic MgO,...
Producing methylcyclopentadiene dimer and trimer based high-performance jet fuels using 5-methyl furfural
Dai, Yiying,Liu, Qing,Liu, Yakun,Liu, Yanan,Ma, Chi,Nie, Genkuo,Pan, Lun,Shi, Chengxiang,Zhang, Xiangwen,Zou, Ji-Jun
supporting information, p. 7765 - 7768 (2020/12/01)
Methylcyclopentadiene dimer and trimer b...
2758-18-1 Process route
-
![N-[3-Methyl-cyclopent-2-en-(Z)-ylidene]-N'-(4-nitro-phenyl)-hydrazine](/upload/2025/12/75812e66-4942-41ee-8843-26e940db51ee.png)
-
88903-26-8
N-[3-Methyl-cyclopent-2-en-(Z)-ylidene]-N'-(4-nitro-phenyl)-hydrazine

-
-
2758-18-1
3-Methyl-2-cyclopenten-1-one

-
-
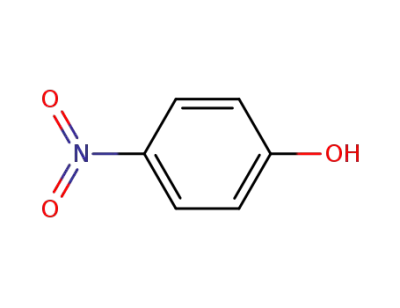
100-02-7,78813-13-5,89830-32-0
4-nitro-phenol

-
-
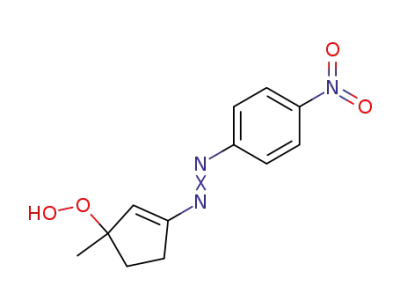
88903-34-8
1-Methyl-3-(4-nitro-phenylazo)-cyclopent-2-enyl-hydroperoxide

-
![2-[(4-Nitro-phenyl)-hydrazono]-5-oxo-hexanal](/upload/2025/12/f6d08d44-c5df-43d6-ae2e-b0e24cc5a75b.png)
-
88903-35-9
2-[(4-Nitro-phenyl)-hydrazono]-5-oxo-hexanal
| Conditions | Yield |
|---|---|
|
With
Co(Saplr); oxygen;
In
dichloromethane;
for 2h;
Further byproducts given;
Ambient temperature;
|
19% 28% |
-
-
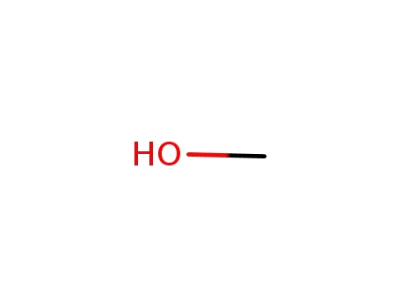
67-56-1
methanol

-
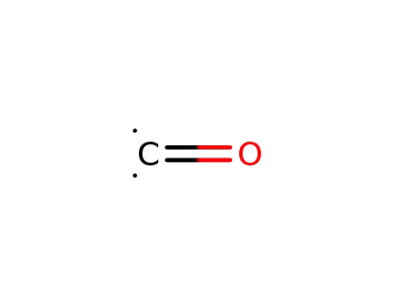
-
201230-82-2
carbon monoxide

-

-
2758-18-1
3-Methyl-2-cyclopenten-1-one

-
-
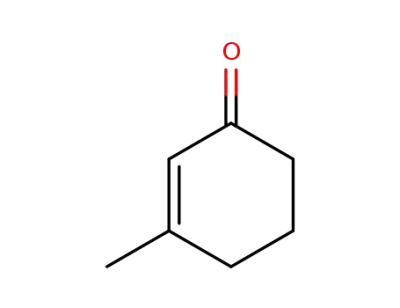
1193-18-6
3-methylcyclohexen-2-one

-
-
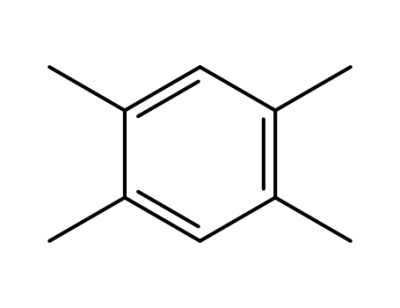
95-93-2
1,2,4,5-tetramethylbenzene

-
-
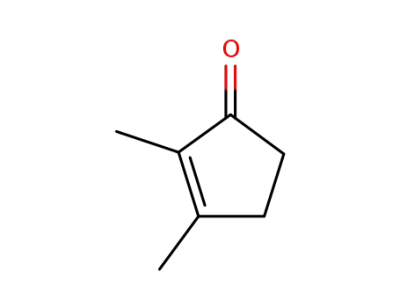
1121-05-7
2,3-dimethylcyclopent-2-enone

-
-
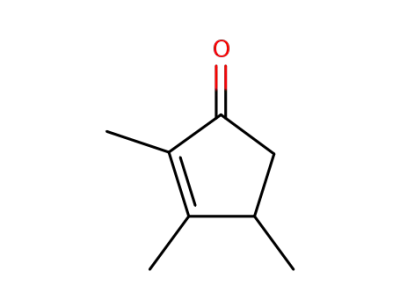
28790-86-5
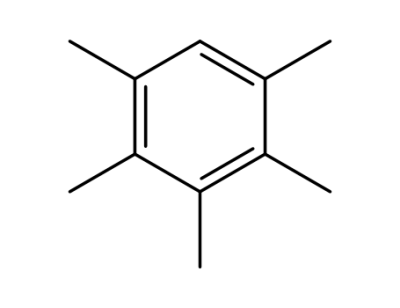
2,3,4-trimethylcyclopent-2-en-1-one

-
-
700-12-9
pentamethylbenzene,

-
-
87-85-4
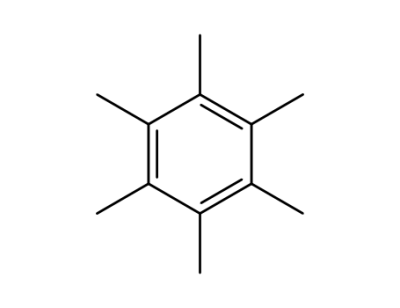
Hexamethylbenzene
| Conditions | Yield |
|---|---|
|
With
acidic H-form ZSM-5 zeolite;
at 249.84 ℃;
under 30003 Torr;
|
2758-18-1 Upstream products
-
41892-81-3
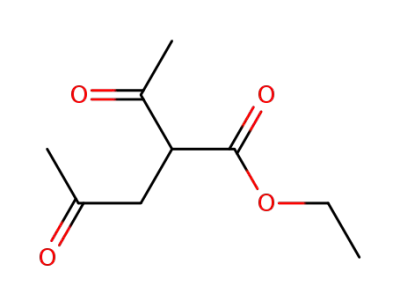
ethyl 2-acetyl-4-oxopentanoate
-
17790-74-8
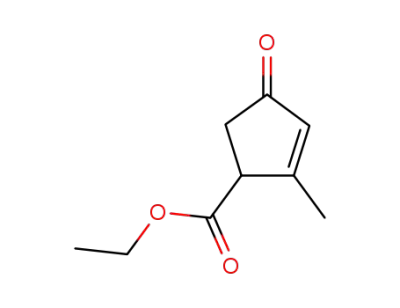
2-methyl-4-oxo-cyclopent-2-enecarboxylic acid ethyl ester
-
854817-78-0
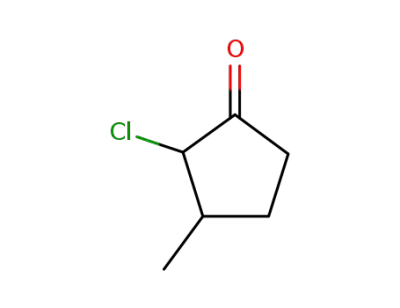
2-chloro-3-methyl-cyclopentanone
-
110-13-4
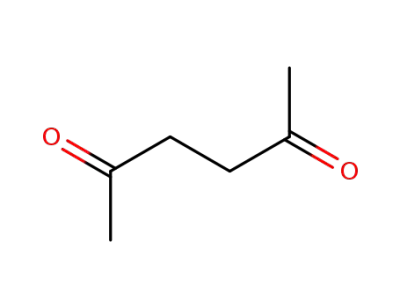
2,5-hexanedione
2758-18-1 Downstream products
-
17024-44-1
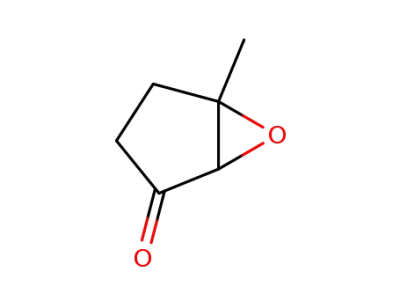
2,3-epoxy-3-methylcyclopentanone
-
859446-70-1
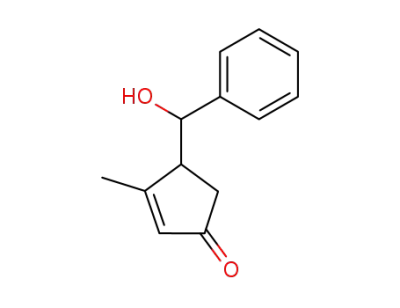
4-(α-hydroxy-benzyl)-3-methyl-cyclopent-2-enone
-
39858-69-0
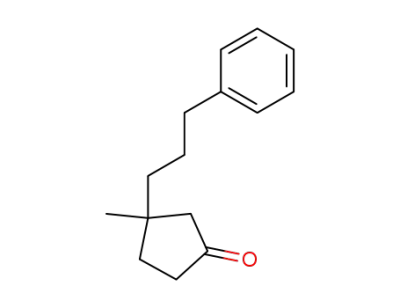
3-methyl-3-(3-phenylpropyl)cyclopentanone
-
80256-22-0
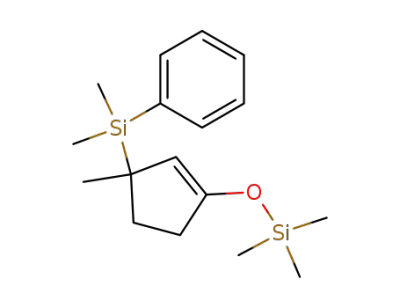
3-(dimethylphenylsilyl)-3-methyl-1-trimethylsilyloxycyclopentene
Relevant Products
-
DIBASIC ESTER
CAS:95481-62-2
-
2-Norbornanemethanol
CAS:5240-72-2
-
5-Norbornene-2-ol
CAS:13080-90-5