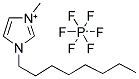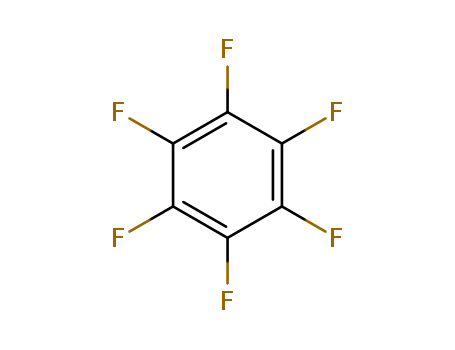
392-56-3
- Product Name:HexafluoroBenzene
- Molecular Formula:C6F6
- Purity:99%
- Molecular Weight:186.056
Product Details;
CasNo: 392-56-3
Molecular Formula: C6F6
Appearance: colourless liquid
factory and supplier 392-56-3 HexafluoroBenzene in stock
- Molecular Formula:C6F6
- Molecular Weight:186.056
- Appearance/Colour:colourless liquid
- Vapor Pressure:94.5mmHg at 25°C
- Melting Point:3.7-4.1 °C(lit.)
- Refractive Index:n20/D 1.377(lit.)
- Boiling Point:80.5 °C at 760 mmHg
- Flash Point:10 °C
- PSA:0.00000
- Density:1.622 g/cm3
- LogP:2.52120
HEXAFLUOROBENZENE(Cas 392-56-3) Usage
|
Chemical Description |
Hexafluorobenzene is a solvent used in the experiments. |
|
Application |
Hexafluorobenzene can react with:Ethyl magnesium bromide in the presence of transition metal halides to form the corresponding perfluoroarylmagnesium compound that can undergo Grignard reactions.The sodium salt of the appropriate phenol in 1,3-dimethyl-2-imidazolidinone (DMEU) to form the corresponding hexakis(aryloxy)benzenes.It can be used:As a ligand to synthesize novel ruthenium(0) and osmium(0) hexafluorobenzene complexes.As a solvent and promoter for the ring-closing metathesis (RCM) to form tetrasubstituted olefins in the presence of a ruthenium-based catalyst. |
|
Definition |
ChEBI: A member of the class of fluorobenzenes that is benzene in which all six hydrogen atom have been replaced by fluorine. |
|
Reactions |
For example, hexafluorobenzene adds chlorine quite readily under rather mild conditions to give hexachlorohexafluorocyclohexane. The catalytic reduction of hexafluorobenzene with hydrogen to penta. and tetra-fluorobenzene at 300 °C, using a platinum catalyst, probably proceeds by a free-radical mechanism. Although the addition of chlorine to hexafluorobenzene is an example of a free-radical addition reaction, the reduction of hexafluorobenzene with hydrogen is classified as a freeradical substitution reaction.One of the earliest and, perhaps, most complicated reactions of hexafluorobenzene is one reported by Desirant. This interesting reaction, whic h is the only example of a high· temperature (above 300°C) reaction of hexafluorobenzene reported to date, involves the pyrolysis of the molecule in a platinum reactor at 850°C. Among the many products produced in this reaction , octafluorotoluene and decafluorobiphenyl were identified. This highly complex reaction probably could also be classified, in some respects, as a free-radical substitution reaction. There is also some less direct evidence that hightemperature reactions of hexafluorobe nzene do occur. In the synthesis of hexafluorobenzene by the pyrolysis of tribromofluoromethane, bromopentafluorobenzene is a signifi'cant by-product. Lesser amounts of higher brominated fluorocarbons are formed as well, along with copious quantities of bromine. This rather complex reaction is illustrated below.CFBr3--630-640℃-->C6F6+Br2+C6F5Br+C6F4Br2+etc. |
|
General Description |
Hexafluorobenzene was repoted to be a sensitive 19F NMR indicator of tumor oxygenation. Rotational Raman spectra of hexafluorobenzenehas been studied under high resolution using a single mode argon laser as the exciting source. Hexafluorobenzene in the gas phase reacts spontaneously with lithium amalgam, to give a solid and intimate mixture of lithium fluoride and elemental polymeric carbon with a small amount of superstoichiometric lithium. Hexafluorobenzene forms series of 1:1 complexes with naphthalene, anthracene,phenanthrene, pyrene and triphenylene. |
|
Hazard |
Toxic by inhalation. Combustible. |
|
Synthesis |
The direct synthesis of hexafluorobenzene from benzene and fluorine is not possible. The synthetic route proceeds via the reaction of alkali-fluorides with halogenated benzene:C6Cl6 + 6 KF → C6F6 + 6 KCl |
|
Purification Methods |
Main impurities are incompletely fluorinated benzenes. Purify it by standing in contact with oleum for 4hours at room temperature, repeating until the oleum does not become coloured. Wash it several times with water, then dry it with P2O5. Finally purify it by repeated fractional crystallisation. [Beilstein 5 III 523, 5 IV 640.] |
InChI:InChI=1/C6F6/c7-1-2(8)4(10)6(12)5(11)3(1)9
392-56-3 Relevant articles
Temperature-enhanced electron detachment from C6F6- negative ions
Datskos, P. G.,Christophorou, L. G.,Carter, J. G.
, p. 7875 - 7882 (1993)
A method is described whereby photoelect...
Perfluorobarrelene
Ralli, Philip,Zhang, Yin,Lemal, David M.
, p. 7349 - 7351 (2008)
The title fluorocarbon has been synthesi...
Laser Photodetachment Spectra of C6F6- in Nonpolar Liquids
Sowada, Ulrich,Holroyd, Richard A.
, p. 1150 - 1154 (1980)
Aromatic anions absorb in the visible re...
Progress toward an absolute gas-phase proton affinity scale
Szulejko,McMahon
, p. 7839 - 7848 (1993)
The temperature dependence of the proton...
Ionizing reaction cross sections in the collision of argon atoms in high Rydberg states with various molecules
Dimicoli, I.,Botter, R.
, p. 2346 - 2354 (1981)
Measurements of absolute ionizing reacti...
Ion-pair formation in the collision of high Rydberg argon atoms with SF6 and C6F6 and negative ion lifetimes
Dimicoli, I.,Botter, R.
, p. 2355 - 2360 (1981)
The ion-pair formation in collision betw...
Magnetic field effects on recombination fluorescence in liquid iso-octane
Saik, Vladimir O.,Ostafin, Agnes E.,Lipsky, Sanford
, p. 7347 - 7358 (1995)
The 123.6 nm photoionization of deuterat...
-
Richardson,T.J.,Bartlett,N.
, p. 427 - 428 (1974)
-
Single and double ionization of corannulene and coronene
Schroeder, Detlef,Loos, Jessica,Schwarz, Helmut,Thissen, Roland,Preda, Dorin V.,Scott, Lawrence T.,Caraiman, Doina,Frach, Maxim V.,Boehme, Dielhard K.
, p. 1625 - 1634 (2001)
Electron-transfer processes that involve...
ELECTROPOLYMERISATION OF PERFLUOROCYCLO-ALKENES.
Briscoe, Mark W.,Chambers, Richard D.,Silvester, Michael J.
, p. 1295 - 1298 (1988)
Novel conducting materials are obtained ...
Laser-Induced Fluorescence Studies of Large and Small Molecular Cations Produced by Using Electron Bombardment in a Free Jet Expansion
Lester, Marsha Isack,Zegarski, B. R.,Miller, Terry A.
, p. 5228 - 5233 (1983)
Laser-induced fluorescence (LIF) excitat...
Stabilities and Structures of C6F6-(C6F6) and C6F6+(C6F6)
Hiraoka, Kenzo,Mizuse, Susumu,Yamabe, Shinichi
, p. 3689 - 3694 (1990)
The equilibria of clustering reactions C...
Facile Synthesis of a Fully Fused, Three-Dimensional ?-Conjugated Archimedean Cage with Magnetically Shielded Cavity
Han, Yi,Jiao, Tianyu,Li, Zhengtao,Ni, Yong,Wu, Jishan,Wu, Shaofei,Zhang, Qiuyu,Zhu, Jun
, p. 14314 - 14321 (2021/09/13)
The synthesis of molecular cages consist...
Preparation method of hexafluorobenzene
-
Paragraph 0024-0031, (2018/05/16)
The invention relates to a preparation m...
Xenon(IV)-carbon bond of [C6F5XeF2]+; Structural characterization and bonding of [C6F5XeF2][BF4], [C6F5XeF2][BF4]·2HF, and [C6F5XeF2][BF4]· n NCCH 3 (n = 1, 2); And the fluorinating properties of [C6F5XeF2][BF4]
Koppe, Karsten,Haner, Jamie,Mercier, Hlne P. A.,Frohn, Hermann-J.,Schrobilgen, Gary J.
, p. 11640 - 11661 (2015/01/16)
The [C6F5XeF2]+ cation is the only examp...
Interaction of the electrophilic bis(pentafluorophenyl)iodonium cation [(C6F5)2I]+ with the ambident pseudohalogenide anions [SCN]- and [CN]-
Hirschberg, Markus E.,Barthen, Peter,Frohn, Hermann-Josef,Bl?ser, Dieter,Tobey, Briac,Jansen, Georg
, p. 28 - 33 (2014/05/20)
The iodonium pseudohalide compounds, [(C...
392-56-3 Process route
-
-
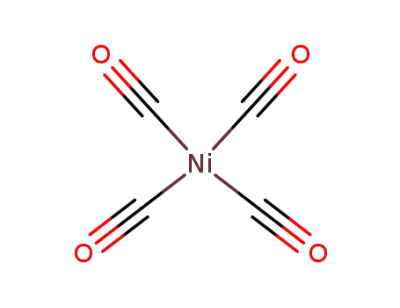
Ni*C6F6

-
-
392-56-3
Hexafluorobenzene

-
-
13463-39-3,71564-36-8
tetracarbonyl nickel
| Conditions | Yield |
|---|---|
|
With
carbon monoxide;
under 0°C;
|
11% 10% |
|
With
CO;
under 0°C;
|
11% 10% |
-

-
53863-36-8
hexafluorobenzene hexafluoroarsenate

-

-
392-56-3
Hexafluorobenzene

-
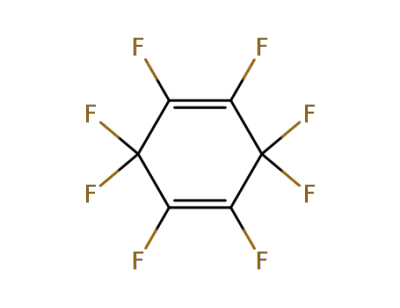
-
775-51-9
octafluorocyclohexa-1,4-diene
| Conditions | Yield |
|---|---|
|
With
cesium fluoride;
In
hydrogen fluoride;
|
392-56-3 Upstream products
-
353-54-8
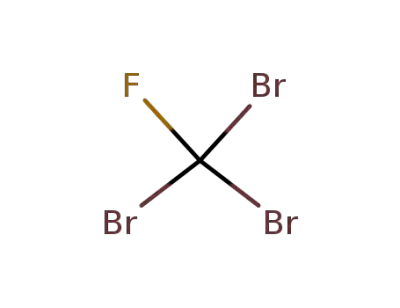
tribromofluoromethane
-
118-74-1
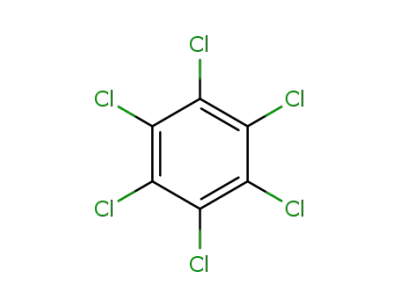
hexachlorobenzene
-
775-51-9

octafluorocyclohexa-1,4-diene
-
356-34-3
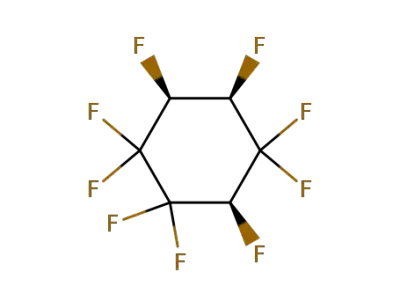
(+/-)-1rH,2cH,4cH-nonafluoro-cyclohexane
392-56-3 Downstream products
-
389-40-2
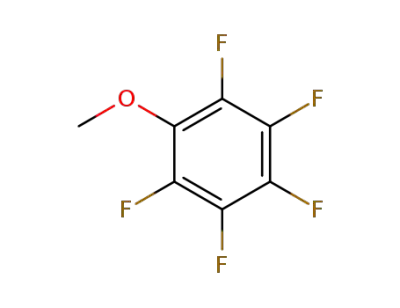
Pentafluoroanisole
-
362-56-1
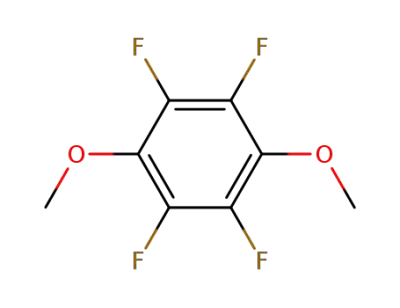
1,2,4,5-tetrafluoro-3,6-dimethoxybenzene
-
771-60-8
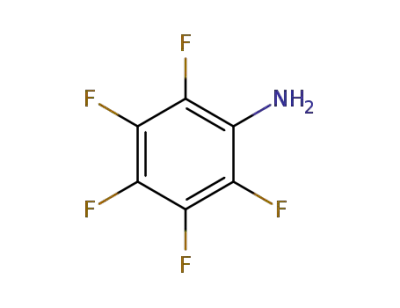
2,3,4,5,6-pentafluoroaniline
-
1535-92-8
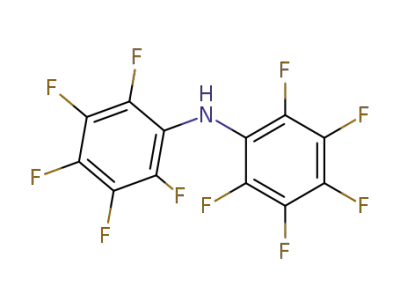
2,2',3,3',4,4',5,5',6,6'-decafluorodiphenylamine
Relevant Products
-
5-Bromo-2-fluoro-m-xylene
CAS:99725-44-7
-
1-Methyl-3-octylimidazolium hexafluorophosphate
CAS:304680-36-2
-
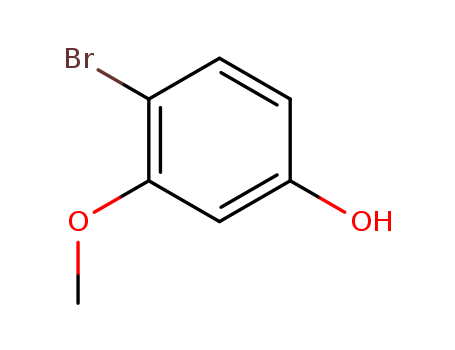
4-Bromo-3-methoxyphenol
CAS:102127-34-4