551-62-2
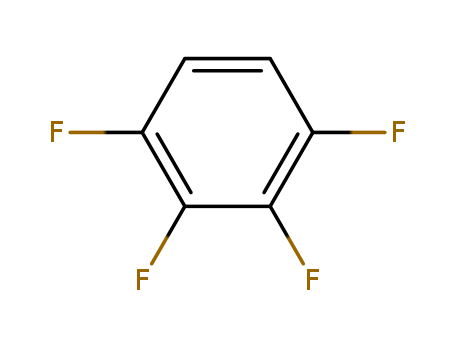
- Product Name:1,2,3,4-Tetrafluorobenzene
- Molecular Formula:C6H2F4
- Purity:99%
- Molecular Weight:150.075
Product Details;
CasNo: 551-62-2
Molecular Formula: C6H2F4
Appearance: colourless liquid
factory and supplier 551-62-2 1,2,3,4-Tetrafluorobenzene in stock
- Molecular Formula:C6H2F4
- Molecular Weight:150.075
- Appearance/Colour:colourless liquid
- Melting Point:-42 °C(lit.)
- Refractive Index:n20/D 1.408(lit.)
- Boiling Point:92.9 °C at 760 mmHg
- Flash Point:20.6 °C
- PSA:0.00000
- Density:1.42 g/cm3
- LogP:2.24300
1,2,3,4-Tetrafluorobenzene(Cas 551-62-2) Usage
InChI:InChI=1/C6H2F4/c7-3-1-2-4(8)6(10)5(3)9/h1-2H
551-62-2 Relevant articles
NHC·Alane Adducts as Hydride Sources in the Hydrodefluorination of Fluoroaromatics and Fluoroolefins
Schneider, Heidi,Hock, Andreas,Jaeger, Alma D.,Lentz, Dieter,Radius, Udo
, p. 4031 - 4043 (2018/09/11)
We present herein the utilization of NHC...
A Predictive Model for the Decarboxylation of Silver Benzoate Complexes Relevant to Decarboxylative Coupling Reactions
Crovak, Robert A.,Hoover, Jessica M.
, p. 2434 - 2437 (2018/02/28)
Decarboxylative coupling reactions offer...
Transition-Metal-Free Catalytic Hydrodefluorination of Polyfluoroarenes by Concerted Nucleophilic Aromatic Substitution with a Hydrosilicate
Kikushima, Kotaro,Grellier, Mary,Ohashi, Masato,Ogoshi, Sensuke
supporting information, p. 16191 - 16196 (2017/11/27)
A transition-metal-free catalytic hydrod...
Base-Catalyzed Aryl-B(OH)2 Protodeboronation Revisited: From Concerted Proton Transfer to Liberation of a Transient Aryl Anion
Cox, Paul A.,Reid, Marc,Leach, Andrew G.,Campbell, Andrew D.,King, Edward J.,Lloyd-Jones, Guy C.
supporting information, p. 13156 - 13165 (2017/09/26)
Pioneering studies by Kuivila, published...
551-62-2 Process route
-
-
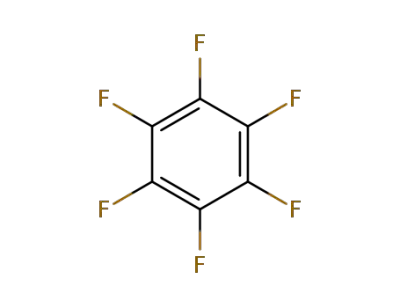
392-56-3
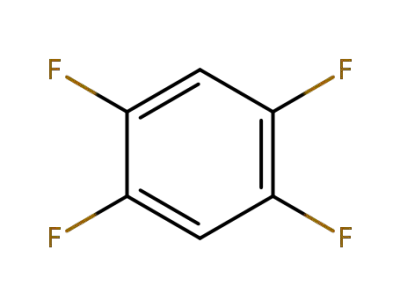
Hexafluorobenzene

-
-
[Rh(μ-H)(1,3-bis(diisopropylphosphanyl)propane)]2

-
-
327-54-8
1,2,4,5-Tetrafluorobenzene

-

-
551-62-2
1,2,3,4-tetrafluorobenzene

-
-
363-72-4
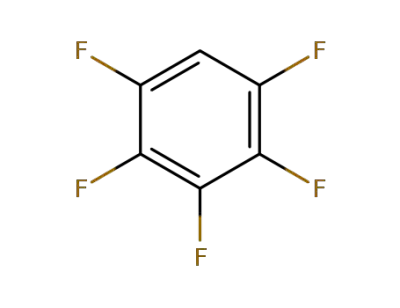
Pentafluorobenzene

-
-
[Rh(μ-F)(1,3-bis(diisopropylphosphanyl)propane)]2
| Conditions | Yield |
|---|---|
|
In
benzene-d6;
at 50 ℃;
for 96h;
regioselective reaction;
Inert atmosphere;
|
-

-
392-56-3
Hexafluorobenzene

-

-
327-54-8
1,2,4,5-Tetrafluorobenzene

-

-
551-62-2
1,2,3,4-tetrafluorobenzene

-

-
363-72-4
Pentafluorobenzene

-
-
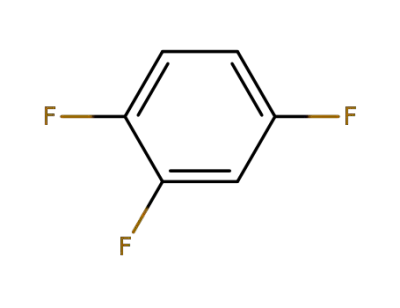
367-23-7
1,2,4-trifluorobenzene
| Conditions | Yield |
|---|---|
|
With
(1,3-dimethylimidazolin-2-ylidene)*AlH3;
In
5,5-dimethyl-1,3-cyclohexadiene;
at 135 ℃;
for 25h;
|
74% 18% 4% 3% |
551-62-2 Upstream products
-
769-37-9
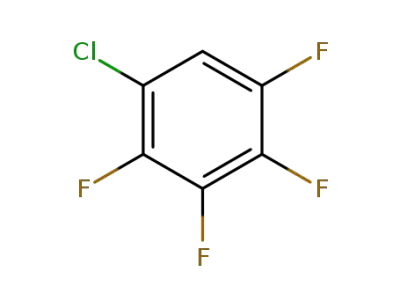
1-chloro-2,3,4,5-tetrafluorobenzene
-
363-72-4

Pentafluorobenzene
-
17065-31-5
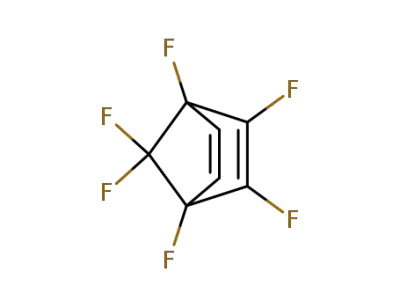
1,2,3,4,7,7-hexafluorobicyclo<2,2,1>hepta-2,5-diene
-
23657-35-4
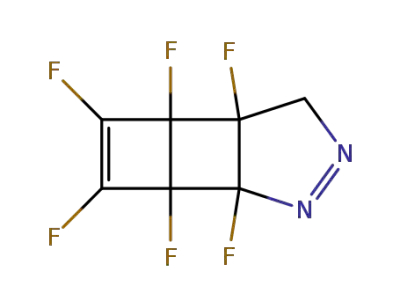
1,2,3,4,5,6-hexafluoro-7,8-diazatricyclo<4.3.0.02,5>nona-3,7-diene
551-62-2 Downstream products
-
2708-97-6
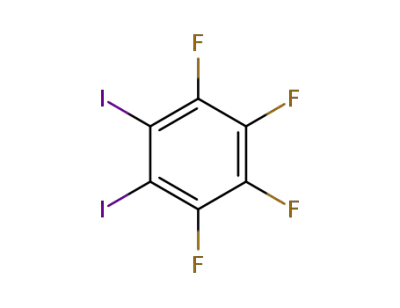
5,6-diiodo-1,2,3,4-tetrafluorobenzene
-
1074-91-5
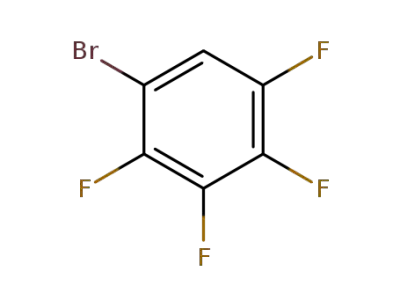
1-bromo-2,3,4,5-tetrafluorobenzene
-
827-08-7
1,2-dibromo-3,4,5,6-tetrafluorobenzene
-
5580-79-0
2,3,4,5-tetrafluoro-1-nitrobenzene
Relevant Products
-
5-Bromo-2-fluoro-m-xylene
CAS:99725-44-7
-
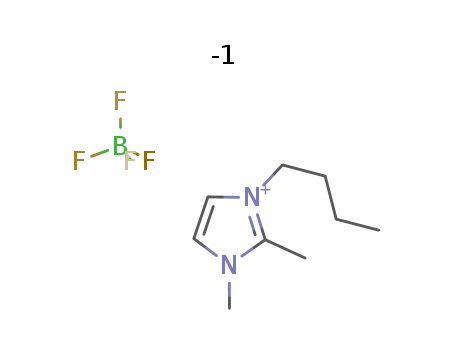
1-butyl-2,3-dimethylimidazolium tetrafluoroborate
CAS:402846-78-0
-
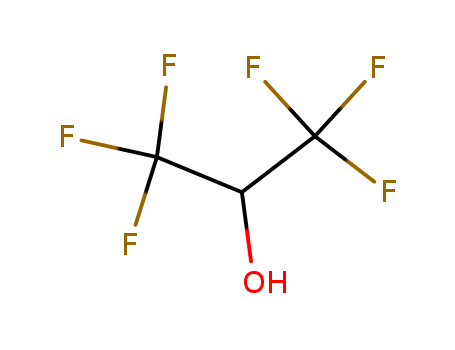
Hexafluoroisopropanol
CAS:920-66-1