86-96-4
- Product Name:Benzoyleneurea
- Molecular Formula:C8H6N2O2
- Purity:99%
- Molecular Weight:162.148
Product Details;
CasNo: 86-96-4
Molecular Formula: C8H6N2O2
Appearance: white to light pink fluffy powder
factory and supplier 86-96-4 Benzoyleneurea in stock
- Molecular Formula:C8H6N2O2
- Molecular Weight:162.148
- Appearance/Colour:white to light pink fluffy powder
- Vapor Pressure:0.015mmHg at 25°C
- Melting Point:300 °C
- Refractive Index:1.565
- Boiling Point:491.9oC at 760 mmHg
- PKA:11.12±0.20(Predicted)
- Flash Point:119.681°C
- PSA:65.72000
- Density:1.337 g/cm3
- LogP:0.21640
Benzoyleneurea(Cas 86-96-4) Usage
|
Synthesis Reference(s) |
Journal of Heterocyclic Chemistry, 21, p. 5, 1984 DOI: 10.1002/jhet.5570210102Organic Syntheses, Coll. Vol. 2, p. 79, 1943 |
InChI:InChI=1/C8H8N2O/c11-8-9-5-6-3-1-2-4-7(6)10-8/h1-4H,5H2,(H2,9,10,11)
86-96-4 Relevant articles
Synthesis of quinazoline-2,4(1H,3H)-dione from carbon dioxide and 2-aminobenzonitrile using mesoporous smectites incorporating alkali hydroxide
Fujita, Shin-Ichiro,Tanaka, Masahiro,Arai, Masahiko
, p. 1563 - 1569 (2014)
A series of magnesium containing mesopor...
Synthetic approaches, functionalization and therapeutic potential of quinazoline and quinazolinone skeletons: The advances continue
Khan, Imtiaz,Ibrar, Aliya,Ahmed, Waqas,Saeed, Aamer
, p. 124 - 169 (2015)
The presence of N-heterocycles as an ess...
-
Lange,Sheibley
, p. 79 (1943)
-
ZIF-8-Nanocrystalline Zirconosilicate Integrated Porous Material for the Activation and Utilization of CO2 in Insertion Reactions
Srivastava, Diksha,Rani, Poonam,Srivastava, Rajendra
, p. 1132 - 1139 (2020)
The conversion of CO2 to useful chemical...
Delineating the Mechanism of Ionic Liquids in the Synthesis of Quinazoline-2,4(1H,3H)-dione from 2-Aminobenzonitrile and CO2
Hulla, Martin,Chamam, Sami M. A.,Laurenczy, Gabor,Das, Shoubhik,Dyson, Paul J.
, p. 10559 - 10563 (2017)
Ionic liquids (ILs) are versatile solven...
-
Hegarty,Bruice
, p. 4924 (1969)
-
UNEXPECTED FORMATION OF 2,4-QUINAZOLINEDIONE IN THE REACTION OF α-CYANO-β-DIMETHYLAMINOCROTONAMIDE WITH ETHYL ANTHRANILATE
Yalysheva, N. Z.,Granik, V. G.
, p. 1186 (1984)
-
Design, synthesis, in silico ADMET, docking, and antiproliferative evaluations of [1,2,4]triazolo[4,3-c]quinazolines as classical DNA intercalators
Alesawy, Mohamed S.,Eissa, Ibrahim H.,El-Adl, Khaled,Ibrahim, Mohamed-Kamal
, (2022/01/13)
Eleven novel [1,2,4]triazolo[4,3-c]quina...
Synthesis, biological evaluation, and molecular docking of new series of antitumor and apoptosis inducers designed as VEGFR-2 inhibitors
Abdallah, Abdallah E.,Abo-Saif, Mariam A.,Al Ward, Maged Mohammed Saleh,Alesawy, Mohamed S.,Eissa, Sally I.,El-Feky, Ola A.,El-Zahabi, Mohamed Ayman,Elkaeed, Eslam B.,Mabrouk, Reda R.,Mehany, Ahmed B. M.
, p. 573 - 591 (2022/01/20)
Based on quinazoline, quinoxaline, and n...
[TBDH][HFIP] ionic liquid catalyzed synthesis of quinazoline-2,4(1H,3H)-diones in the presence of ambient temperature and pressure
Phatake, Vishal V.,Gokhale, Tejas A.,Bhanage, Bhalchandra M.
, (2021/07/28)
The utilization of carbon dioxide under ...
Synthesis of acyclic nucleoside phosphonates targeting flavin-dependent thymidylate synthase in Mycobacterium tuberculosis
Agrofoglio, Luigi A.,Becker, Hubert F.,Biteau, Nicolas G.,Lambry, Jean-Christophe,Myllykallio, Hannu,Roy, Vincent
, (2021/08/16)
Flavin-Dependent Thymidylate Synthase (F...
86-96-4 Process route
-
-
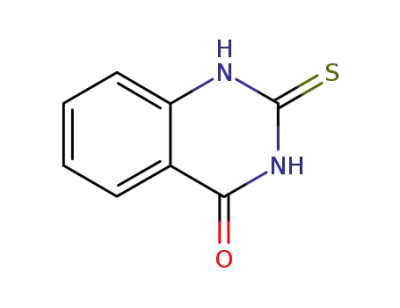
13906-09-7
2-thioxo-2,3-dihydro-1H-quinazolin-4-one

-
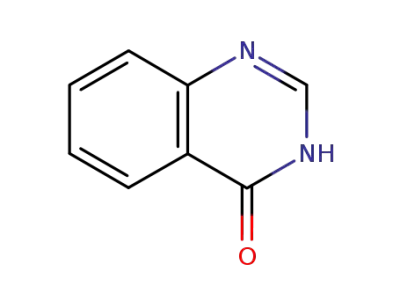
-
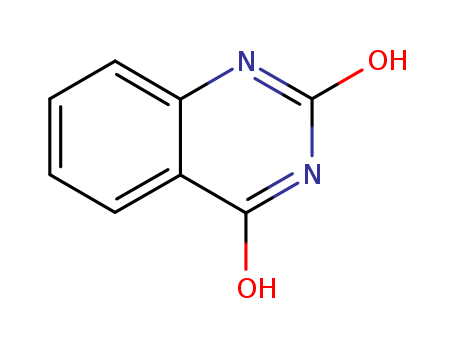
491-36-1
4-Hydroxyquinazoline

-
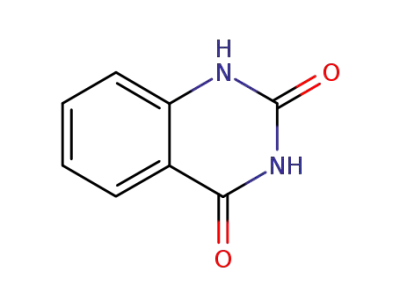
-
86-96-4
1,2,3,4-tetrahydro-quinazoline-2,4-dione
| Conditions | Yield |
|---|---|
|
With
ozone;
In
water; acetic acid;
at 25 ℃;
for 1h;
|
13% 75% |
-
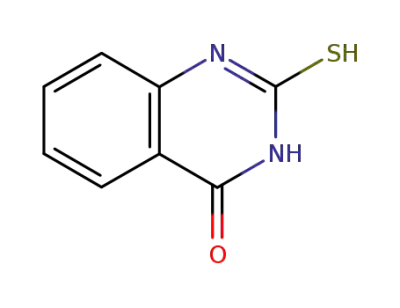
-
13906-09-7
2-mercapto-3H-quinazolin-4-one

-

-
491-36-1
4-Hydroxyquinazoline

-

-
86-96-4
1,2,3,4-tetrahydro-quinazoline-2,4-dione
| Conditions | Yield |
|---|---|
|
With
ozone;
In
water; acetic acid;
at 25 ℃;
for 1h;
|
13% 75% |
86-96-4 Upstream products
-
491-36-1

4-Hydroxyquinazoline
-
612-53-3
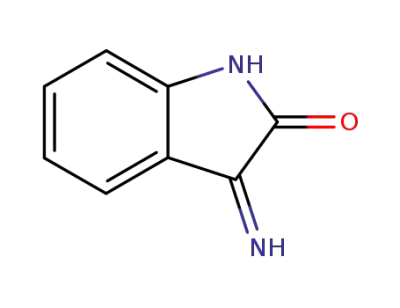
(E)-3-iminoindolin-2-one
-
88-96-0
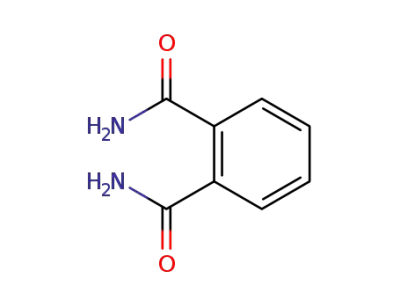
phthalamide
-
57212-70-1
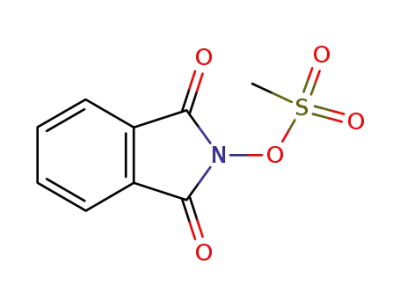
N-(mesyloxy)phthalimide
86-96-4 Downstream products
-
607-19-2
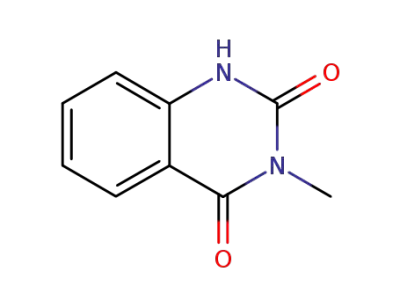
3-methyl-2,4(1H,3H)-quinazolinedione
-
1013-01-0
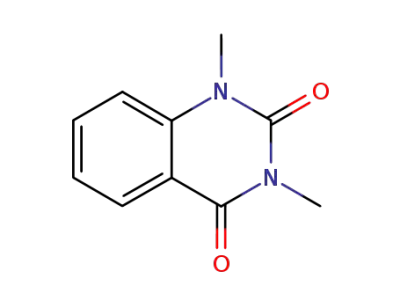
1,3-dimethyl-1H-quinazoline-2,4-dione
-
84587-34-8
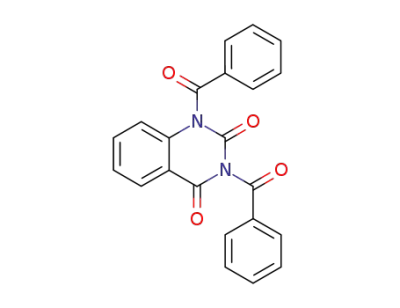
1,3-dibenzoylquinazoline-2,4-dione
-
2217-26-7
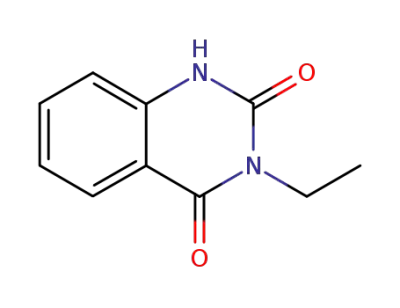
3-ethyl-1H-quinazoline-2,4-dione
Relevant Products
-
2,5-Dimethoxy-Beta-Nitrostyrene
CAS:40276-11-7
-
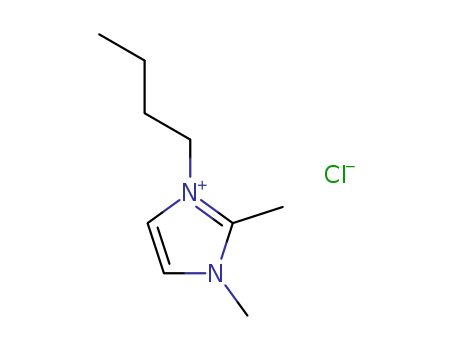
1-Butyl-2,3-Dimethylimidazolium Chloride
CAS:98892-75-2
-
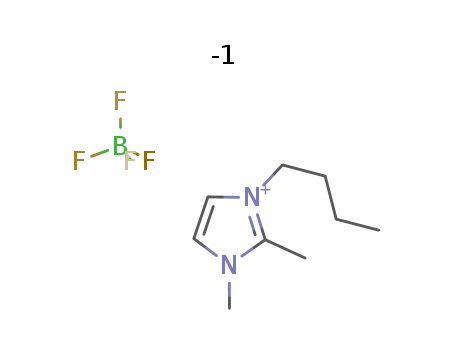
1-butyl-2,3-dimethylimidazolium tetrafluoroborate
CAS:402846-78-0