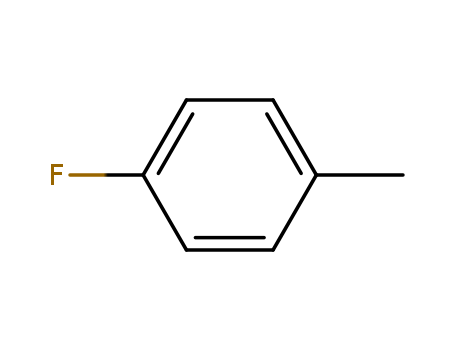
352-32-9
- Product Name:4-Fluorotoluene
- Molecular Formula:C7H7F
- Purity:99%
- Molecular Weight:110.131
Product Details;
CasNo: 352-32-9
Molecular Formula: C7H7F
Appearance: clear colorless to slightly yellow liquid
factory and supplier 352-32-9 4-Fluorotoluene in stock
- Molecular Formula:C7H7F
- Molecular Weight:110.131
- Appearance/Colour:clear colorless to slightly yellow liquid
- Vapor Pressure:21.1mmHg at 25°C
- Melting Point:-56 °C
- Refractive Index:n20/D 1.468(lit.)
- Boiling Point:117 °C at 760 mmHg
- Flash Point:11.9 °C
- PSA:0.00000
- Density:1.001 g/cm3
- LogP:2.13410
p-Fluorotoluene(Cas 352-32-9) Usage
|
Air & Water Reactions |
Highly flammable. |
|
Reactivity Profile |
p-Fluorotoluene may be incompatible with strong oxidizing and reducing agents. May be incompatible with amines, nitrides, azo/diazo compounds, alkali metals, and epoxides. Products of combustion contain toxic fluoride fumes. |
|
Fire Hazard |
Special Hazards of Combustion Products: Toxic fumes of fluoride |
|
Safety Profile |
Moderately toxic by parenteral route. A very dangerous fire hazard when exposed to heat or flame; can react vigorously with oxibzing materials. When heated to decomposition it emits toxic fumes of F-. See also FLUORIDES. |
|
Purification Methods |
Purify it as for o-fluorotoluene. [Beilstein 5 H 290, 5 III 677, 5 IV 799.] |
|
General Description |
A colorless liquid with an aromatic odor. May float or sink in water. |
InChI:InChI=1/C7H7F/c1-6-2-4-7(8)5-3-6/h2-5H,1H3
352-32-9 Relevant articles
Liquid-phase fluorination of aromatic compounds by elemental fluorine
Conte, L.,Gambaretto, G. P.,Napoli, M.,Fraccaro, C.,Legnaro, E.
, p. 175 - 180 (1995)
The fluorination of aromatic compounds (...
Direct fluorination of toluene using elemental fluorine in gas/liquid microreactors
J?hnisch,Baerns,Hessel,Ehrfeld,Haverkamp,L?we,Wille,Guber
, p. 117 - 128 (2000)
Direct fluorination of toluene, pure or ...
Gas-phase alkylation of fluorobenzene and substituted fluorobenzene by (CH3)2F+ ions
Attina,Ricci
, p. 6775 - 6778 (1991)
The gas-phase methylation of selected fl...
Exhaustive chlorination of [B12H12]2- without chlorine gas and the use of [B12Cl12]2- as a supporting anion in catalytic hydrodefluorination of aliphatic C-F bonds
Gu, Weixing,Ozerov, Oleg V.
, p. 2726 - 2728 (2011)
The fully chlorinated closo-dodecaborate...
Full continuous flow synthesis process of fluorine-containing aromatic hydrocarbon compounds
-
Paragraph 0081-0094, (2021/04/07)
The invention provides a full continuous...
A Mild, General, Metal-Free Method for Desulfurization of Thiols and Disulfides Induced by Visible-Light
Qiu, Wenting,Shi, Shuai,Li, Ruining,Lin, Xianfeng,Rao, Liangming,Sun, Zhankui
supporting information, p. 1255 - 1258 (2021/05/05)
A visible-light-induced metal-free desul...
Coupling Photocatalysis and Substitution Chemistry to Expand and Normalize Redox-Active Halides
Rathnayake, Manjula D.,Weaver, Jimmie D.
supporting information, p. 2036 - 2041 (2021/04/05)
Photocatalysis can generate radicals in ...
Hydrodehalogenation of organohalides by Et3SiH catalysed by group 4 metal complexes and B(C6F5)3
?ilková, Nadě?da,Dunlop, David,Horá?ek, Michal,Lama?, Martin,Pinkas, Ji?í
supporting information, p. 2771 - 2775 (2020/03/13)
Catalytic hydrodehalogenation (HDH) of a...
352-32-9 Process route
-
-
o-tolyl(p-tolyl)iodonium trifluoromethanesulfonate

-
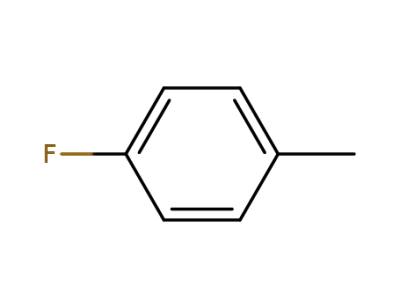
-
352-32-9
p-fluorotoluene

-
-
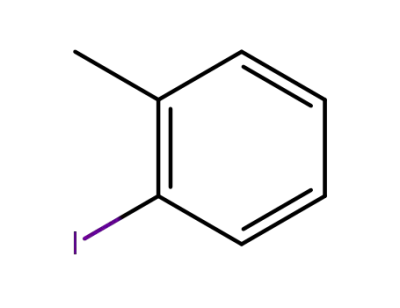
615-37-2
ortho-methylphenyl iodide

-
-
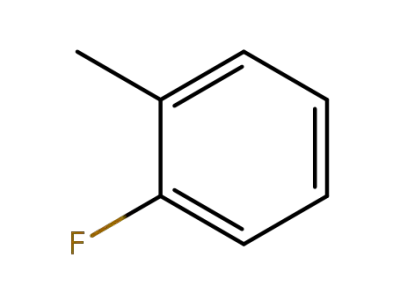
95-52-3
2-Fluorotoluene

-
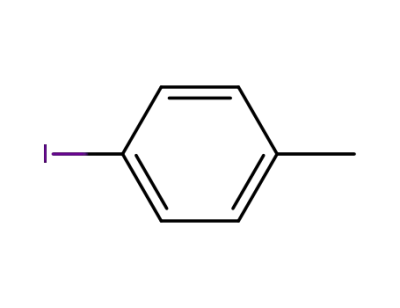
-
624-31-7
4-tolyl iodide

-
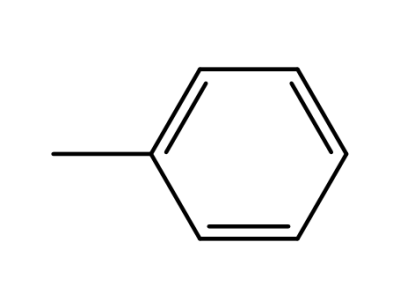
-
108-88-3,15644-74-3,16713-13-6
toluene
| Conditions | Yield |
|---|---|
|
With
cesium fluoride;
In
acetonitrile;
at 85 ℃;
for 0.666667h;
GC-MS estimated relative yields;
|
-
![4-methylphenyliodonium-(5-[2,2-dimethyl-1,3-dioxane-4,6-dione])ylide](/upload/2026/1/f8a8a3f3-113b-4e18-97bc-262a187522a5.png)
-
1250409-78-9
4-methylphenyliodonium-(5-[2,2-dimethyl-1,3-dioxane-4,6-dione])ylide

-
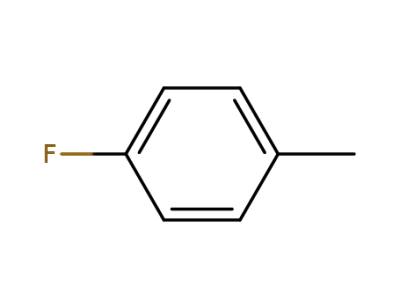
-
352-32-9
p-fluorotoluene

-
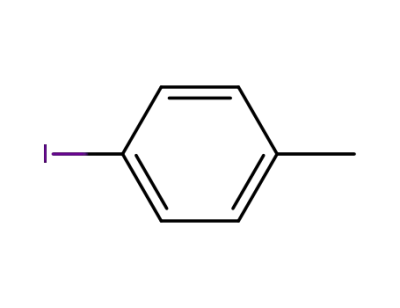
-
624-31-7
4-tolyl iodide

-

-
108-88-3,15644-74-3,16713-13-6
toluene
| Conditions | Yield |
|---|---|
|
With
potassium fluoride; [2.2.2]cryptande;
In
N,N-dimethyl-formamide;
at 130 ℃;
for 0.25h;
Reactivity;
sealed tube;
|
352-32-9 Upstream products
-
2028-84-4

p-methylbenzenediazonium chloride
-
38258-26-3
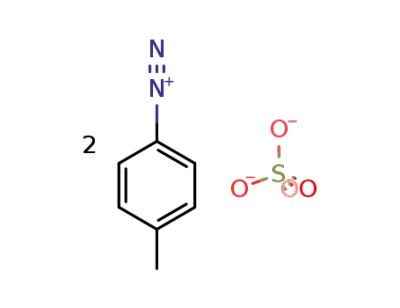
toluene-4-diazonium ; sulfate
-
462-06-6
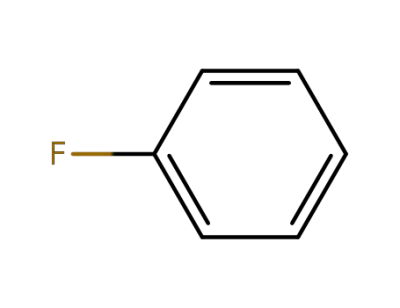
fluorobenzene
-
593-53-3
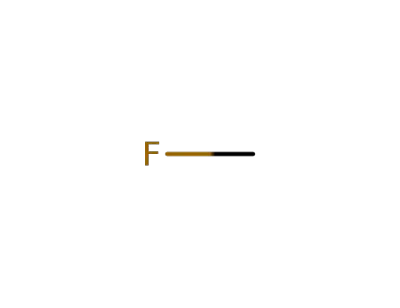
Methyl fluoride
352-32-9 Downstream products
-
342-45-0
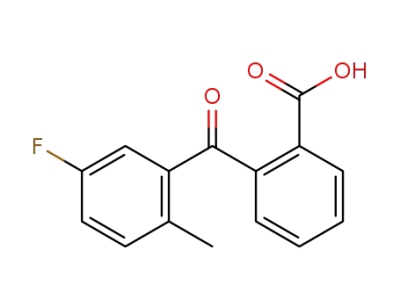
2-(5-fluoro-2-methyl-benzoyl)-benzoic acid
-
319-83-5
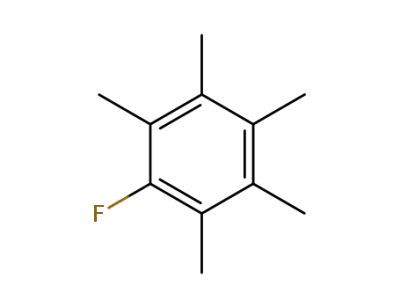
pentamethylfluorobenzene
-
452-62-0
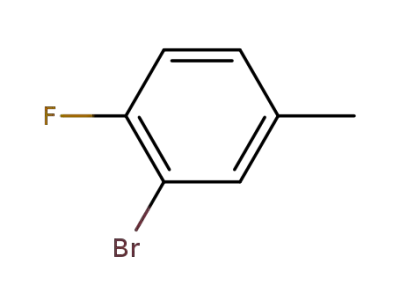
3-bromo-4-fluorotoluene
-
119-33-5
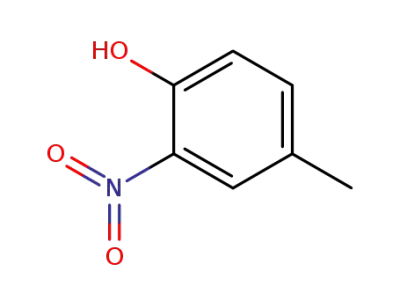
4-methyl-2-nitrophenol
Relevant Products
-
5-Bromo-2-fluoro-m-xylene
CAS:99725-44-7
-
Dicyclohexylphenylphosphine
CAS:6476-37-5
-
2,4-Dimethylaniline
CAS:95-68-1