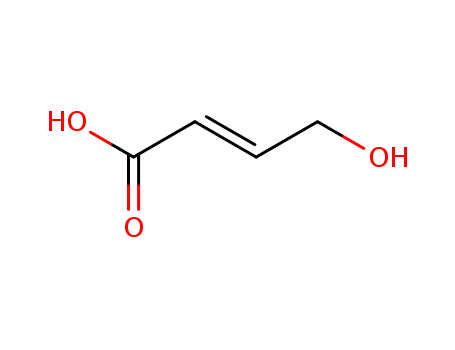
24587-49-3
- Product Name:(E)-4-Hydroxy-2-butenoic Acid
- Molecular Formula:C4H6O3
- Purity:99%
- Molecular Weight:102.09
Product Details;
CasNo: 24587-49-3
Molecular Formula: C4H6O3
factory and supplier 24587-49-3 (E)-4-Hydroxy-2-butenoic Acid in stock
- Molecular Formula:C4H6O3
- Molecular Weight:102.09
- Vapor Pressure:6.49E-06mmHg at 25°C
- Melting Point:109℃
- Boiling Point:338.7oC at 760 mmHg
- PKA:4.31±0.10(Predicted)
- Flash Point:172.9oC
- PSA:57.53000
- Density:1.271±0.06 g/cm3(Predicted)
- LogP:-0.38050
4-HYDROXY-BUT-2-ENOIC ACID(Cas 24587-49-3) Usage
|
Biological Activity |
Binds to the γ -hydroxybutyric acid (GHB) receptor with higher affinity than GHB itself.? May be an endogenous ligand. |
InChI:InChI=1/C4H6O3/c5-3-1-2-4(6)7/h1-2,5H,3H2,(H,6,7)/p-1/b2-1+
24587-49-3 Relevant articles
MODIFIED PHOTOBEHAVIOR OF CARBOXYLIC ACID DERIVATIVES INDUCED BY PROTONATION
Amat, Ana M.,Asensio, Gregorio,Castello, Maria J.,Miranda, Miguel A.,Simon-Fuentes, Antonio
, p. 905 - 910 (1987)
A series of carboxylic acid derivatives ...
INHIBITORS OF BRUTON'S TYROSINE KINASE AND METHODS OF THEIR USE
-
Page/Page column 150, (2018/06/30)
Compounds of formula (I') and methods of...
INHIBITORS OF BRUTON'S TYROSINE KINASE AND METHODS OF THEIR USE
-
Page/Page column 150, (2017/09/02)
The present disclosure is directed to co...
PROCESS FOR THE PRODUCTION OF CARNITINE BY CYCLOADDITION
-
Page/Page column 10, (2012/02/03)
The invention relates to a method for th...
4-Hyroxybutyric acid (and analogues) derivatives of d-glucosamine
Dardoize,Goasdoue,Goadoue,Laborit,Topall
, p. 7783 - 7794 (2007/10/02)
-
24587-49-3 Process route
-
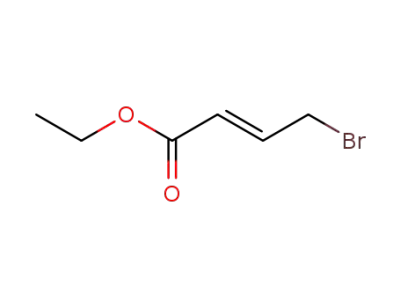
-
37746-78-4
4-bromo-trans-crotonic acid ethyl ester

-
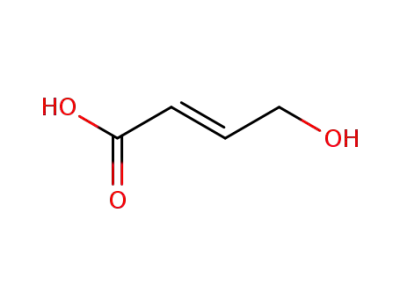
-
24587-49-3
(2E)-4-hydroxybut-2-enoic acid
| Conditions | Yield |
|---|---|
|
With
water; potassium hydroxide;
at 100 ℃;
for 2h;
|
53% |
|
With
water; potassium hydroxide;
at 100 ℃;
for 2h;
|
53% |
|
With
potassium carbonate;
at 80 ℃;
|
-
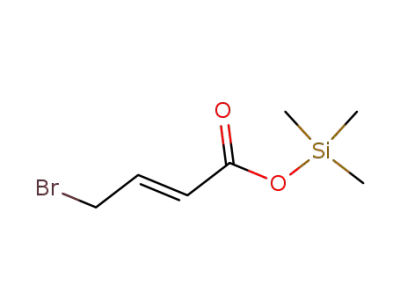
-
88239-39-8
γ-bromocrotonate de trimethylsilyle

-

-
24587-49-3
(2E)-4-hydroxybut-2-enoic acid
| Conditions | Yield |
|---|---|
|
With
potassium hydroxide;
In
water;
at 100 ℃;
for 0.0833333h;
|
80% |
24587-49-3 Upstream products
-
86728-85-0
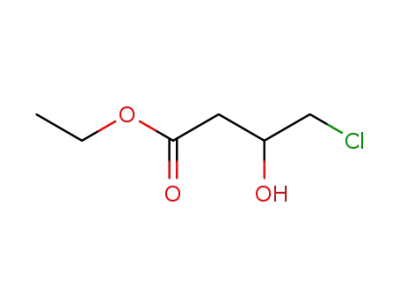
ethyl 4-chloro-3-hydroxybutanoate
-
26805-40-3

γ-acetoxycrotonic acid ethyl ester
-
37746-78-4

4-bromo-trans-crotonic acid ethyl ester
-
28046-78-8
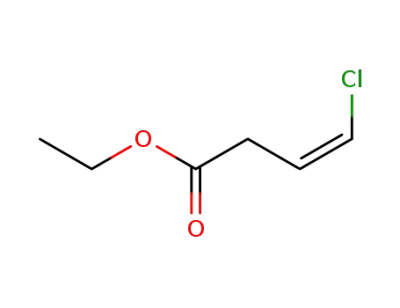
ethyl β-chlorovinylacetate
24587-49-3 Downstream products
-
10080-68-9
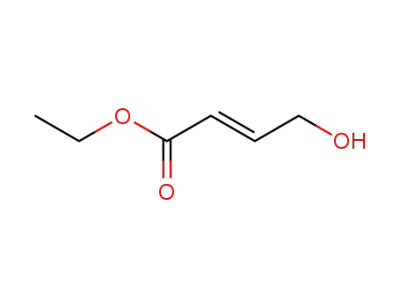
(E)-ethyl 4-hydroxybut-2-enoate
-
26340-58-9
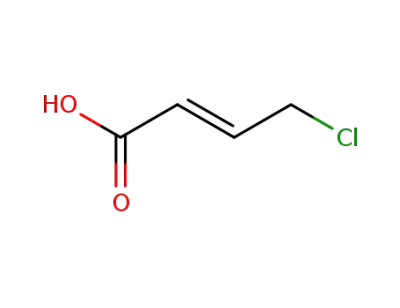
(E)-4-chlorobut-2-enoic acid
-
488-16-4
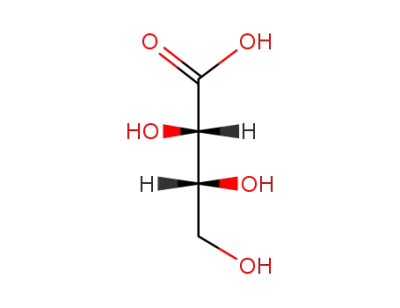
threonic acid
-
108772-86-7
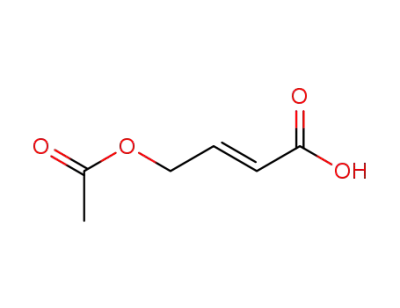
4-acetoxybutene 2-(E) oic acid
Relevant Products
-
DIBASIC ESTER
CAS:95481-62-2
-
1-Bromo-2,4-dichlorobenzene
CAS:1193-72-2
-
2-Fluoro-4-(methylsulfonyl)aniline
CAS:832755-13-2