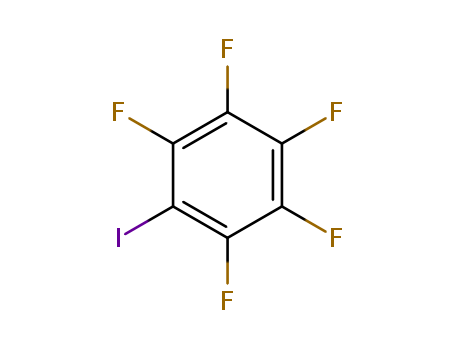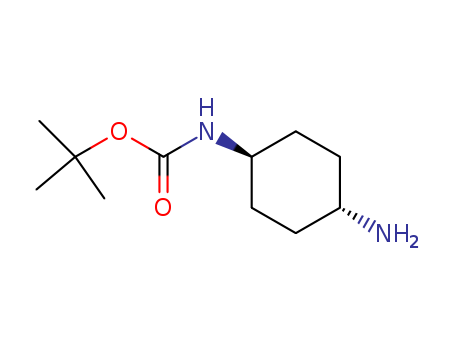
177906-48-8
- Product Name:1-N-Boc-trans-1,4-cyclohexyldiamine
- Molecular Formula:C11H22N2O2
- Purity:99%
- Molecular Weight:214.308
Product Details;
CasNo: 177906-48-8
Molecular Formula: C11H22N2O2
factory and supplier 177906-48-8 1-N-Boc-trans-1,4-cyclohexyldiamine in stock
- Molecular Formula:C11H22N2O2
- Molecular Weight:214.308
- Refractive Index:1.488
- Boiling Point:322.073 °C at 760 mmHg
- PKA:12.44±0.40(Predicted)
- Flash Point:148.585 °C
- PSA:64.35000
- Density:1.029 g/cm3
- LogP:2.87220
TRANS-N-BOC-1,4-CYCLOHEXANEDIAMINE(Cas 177906-48-8) Usage
InChI:InChI=1/C11H22N2O2/c1-11(2,3)15-10(14)13-9-6-4-8(12)5-7-9/h8-9H,4-7,12H2,1-3H3,(H,13,14)/t8-,9-
177906-48-8 Relevant articles
A practical chromatography-free synthesis of a 5,6-dihydroimidazolo[1,5-f]pteridine derivative as a polo-like kinase-1 inhibitor
Ishimoto, Kazuhisa,Nakaoka, Keiichiro,Yabe, Osamu,Nishiguchi, Atsuko,Ikemoto, Tomomi
, p. 5779 - 5790 (2018)
A practical chromatography-free synthesi...
Preparation method of N-Boc-trans-cyclohexanediamine
-
Paragraph 0022-0024, (2021/10/13)
The invention discloses a preparation me...
Preparation method of N-Boc-trans-1,4-cyclohexanediamine
-
Paragraph 0039-0044, (2021/06/06)
The invention discloses a preparation me...
Substituted-3H-imidazo[4,5-c]pyridine and 1H-pyrrolo[2,3-c]pyridine series of novel Ectonucleotide Pyrophosphatase/Phosphodiesterase-1 (ENPP1) and Stimulator for Interferon Genes (STING) modulators as cancer immunotherapeutics
-
Paragraph 0417-0418, (2020/02/19)
Substituted -3H-imidazo[4,5-c]pyridine a...
Smooth receptor ligand
-
Paragraph 0085; 0202; 0205; 0214, (2020/04/01)
The invention relates to the technical f...
177906-48-8 Process route
-
-
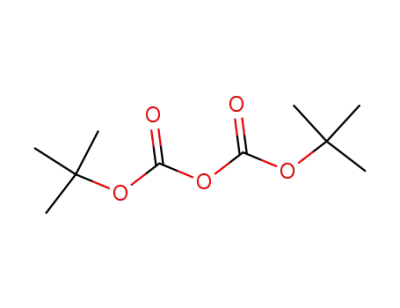
24424-99-5
di-tert-butyl dicarbonate

-
-
2615-25-0
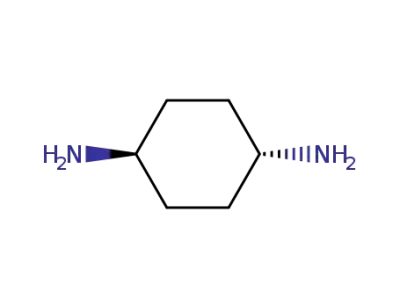
trans-1,4-cyclohexyldiamine

-
-
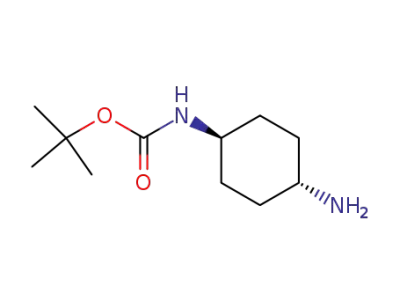
177906-48-8
trans-N-Boc-1,4-cyclohexanediamine
| Conditions | Yield |
|---|---|
|
With
triethylamine;
In
N,N-dimethyl-formamide;
at 20 ℃;
|
86% |
|
In
methanol;
at 0 - 20 ℃;
for 16h;
|
86% |
|
In
dichloromethane;
at 20 ℃;
for 2h;
|
85% |
|
In
methanol;
at 20 ℃;
|
83% |
|
In
chloroform;
for 15h;
|
77% |
|
In
diethyl ether;
at 5 - 20 ℃;
|
66% |
|
In
tetrahydrofuran;
at 0 - 20 ℃;
for 16.5h;
|
43% |
|
In
chloroform;
at 80 ℃;
for 16h;
|
39.1% |
|
In
methanol;
at -60 - 20 ℃;
for 13.5h;
|
27.7% |
|
In
acetonitrile;
at 22 ℃;
for 12h;
|
21% |
|
With
N-ethyl-N,N-diisopropylamine;
In
dichloromethane;
at 20 ℃;
for 24h;
Inert atmosphere;
|
18% |
|
In
chloroform;
at 20 ℃;
|
|
|
With
hydrogenchloride;
In
methanol; water;
at 20 ℃;
for 1h;
|
|
|
In
diethyl ether;
at 20 ℃;
for 1h;
|
|
|
In
water; tert-butyl alcohol;
at 20 ℃;
for 2h;
|
|
|
In
dichloromethane;
at 0 - 20 ℃;
for 16h;
|
|
|
With
hydrogenchloride;
In
ethanol;
|
|
|
With
sodium hydrogencarbonate;
In
tetrahydrofuran;
at 20 ℃;
|
1.4 g |
-
-
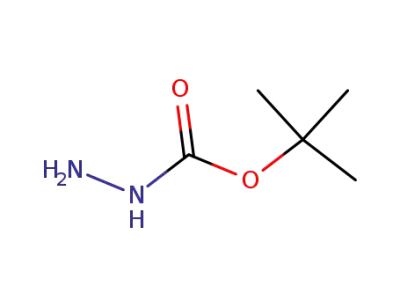
870-46-2
t-butoxycarbonylhydrazine

-

-
2615-25-0
trans-1,4-cyclohexyldiamine

-

-
177906-48-8
trans-N-Boc-1,4-cyclohexanediamine
| Conditions | Yield |
|---|---|
|
With
dihydrogen peroxide; copper dichloride;
In
acetone;
at 10 - 15 ℃;
for 10h;
Inert atmosphere;
|
80.4% |
177906-48-8 Upstream products
-
24424-99-5

di-tert-butyl dicarbonate
-
2615-25-0

trans-1,4-cyclohexyldiamine
-
870-46-2

t-butoxycarbonylhydrazine
177906-48-8 Downstream products
-
251947-23-6
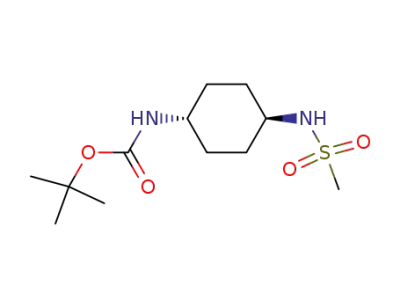
trans-(4-methanesulfonylamino-cyclohexyl)-carbamic acid tert-butyl ester
-
873537-67-8
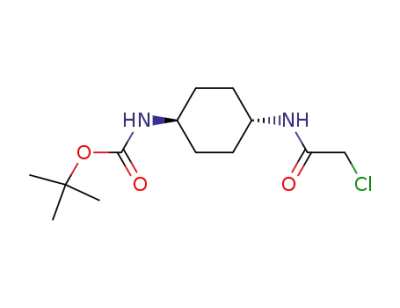
trans-[4-(2-chloroacetylamido)cyclohexyl]carbamic acid tert-butyl ester
-
296270-92-3
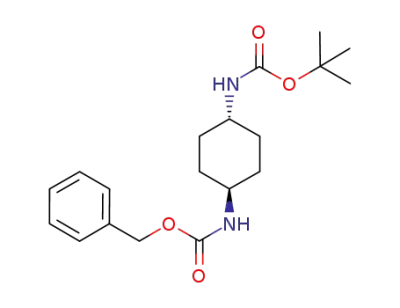
tert-butyl trans-4-(Cbz-amino)cyclohexylcarbamate
Relevant Products
-
2,5-Dimethoxy-Beta-Nitrostyrene
CAS:40276-11-7
-
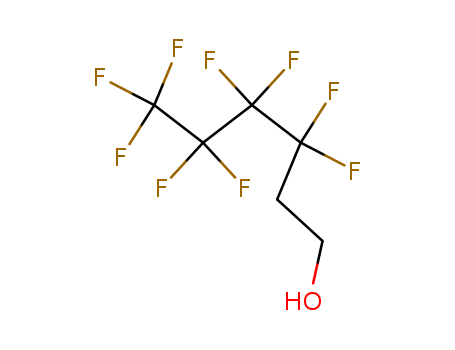
2-Perfluorobutyl ethyl alcohol
CAS:2043-47-2
-
Iodopentafluorobenzene
CAS:827-15-6