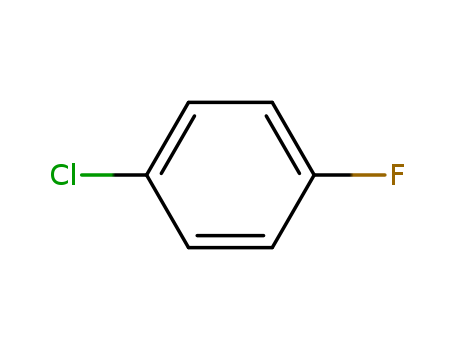
352-33-0
- Product Name:1-Chloro-4-fluorobenzene
- Molecular Formula:C6H4ClF
- Purity:99%
- Molecular Weight:130.549
Product Details;
CasNo: 352-33-0
Molecular Formula: C6H4ClF
Appearance: Clear colourless to light yellow liquid
factory and supplier 352-33-0 1-Chloro-4-fluorobenzene in stock
- Molecular Formula:C6H4ClF
- Molecular Weight:130.549
- Appearance/Colour:Clear colourless to light yellow liquid
- Vapor Pressure:1.04mmHg at 25°C
- Melting Point:-21.5 °C
- Refractive Index:n20/D 1.495(lit.)
- Boiling Point:132.5 °C at 760 mmHg
- Flash Point:27.4 °C
- PSA:0.00000
- Density:1.236 g/cm3
- LogP:2.47910
1-Chloro-4-fluorobenzene(Cas 352-33-0) Usage
InChI:InChI=1/C6H4FI/c7-5-1-3-6(8)4-2-5/h1-4H
352-33-0 Relevant articles
Photocatalytic monofluorination of benzene by fluoride via photoinduced electron transfer with 3-cyano-1-methylquinolinium
Ohkubo, Kei,Fujimoto, Atsushi,Fukuzumi, Shunichi
, p. 10719 - 10725 (2013)
The photocatalytic fluorination of benze...
Nucleophilic Fluorination of Heteroaryl Chlorides and Aryl Triflates Enabled by Cooperative Catalysis
Hong, Cynthia M.,Whittaker, Aaron M.,Schultz, Danielle M.
, p. 3999 - 4006 (2021/03/09)
Aryl and heteroaryl fluorides are growin...
Fluorination of arylboronic esters enabled by bismuth redox catalysis
Planas, Oriol,Wang, Feng,Leutzsch, Markus,Cornella, Josep
, p. 313 - 317 (2020/01/28)
Bismuth catalysis has traditionally reli...
Palladium-catalysed electrophilic aromatic C-H fluorination
Yamamoto, Kumiko,Li, Jiakun,Garber, Jeffrey A. O.,Rolfes, Julian D.,Boursalian, Gregory B.,Borghs, Jannik C.,Genicot, Christophe,Jacq, Jér?me,Van Gastel, Maurice,Neese, Frank,Ritter, Tobias
, p. 511 - 514 (2018/03/02)
Aryl fluorides are widely used in the ph...
Highly efficient Sandmeyer reaction on immobilized CuI/CuII-based catalysts
Tarkhanova, Irina G.,Gantman, Michail G.,Sigeev, Alexander S.,Maslakov, Konstantin I.,Zelikman, Vladimir M.,Beletskaya, Irina P.
, p. 261 - 263 (2018/06/01)
Highly effective embodiment of Sandmeyer...
352-33-0 Process route
-
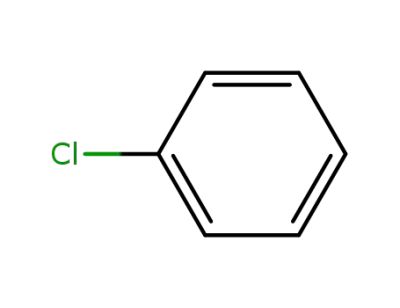
-
108-90-7
chlorobenzene

-
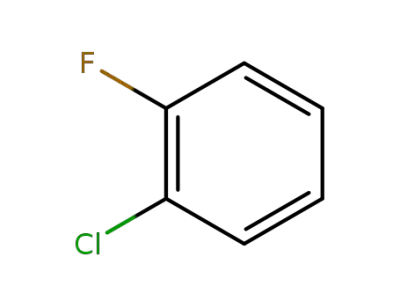
-
348-51-6
1-chloro-2-fluorobenzene

-
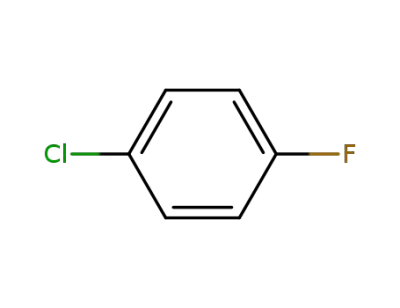
-
352-33-0
1-Chloro-4-fluorobenzene

-
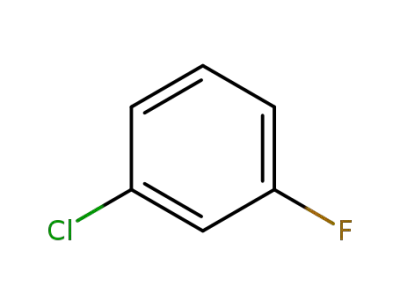
-
625-98-9
3-chlorofluorobenzene

-
-
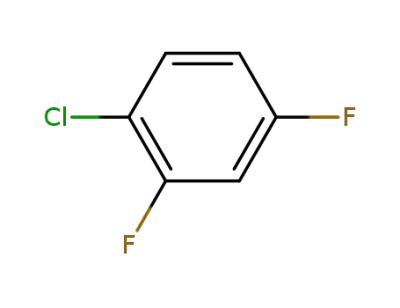
1435-44-5
1-chloro-2,4-difluorobenzene

-
-
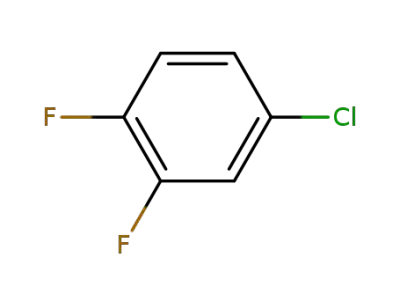
696-02-6
4-chloro-1,2-difluorobenzene

-
-
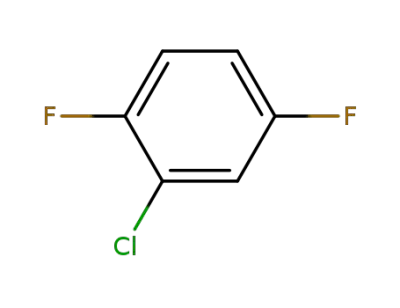
2367-91-1
1-chloro-2,5-difluorobenzene
| Conditions | Yield |
|---|---|
|
With
xenon difluoride; boron trifluoride diethyl etherate;
at 0 - 25 ℃;
Reagent/catalyst;
Cooling with ice;
|
0.08 mmol 0.02 mmol 0.31 mmol 0.01 mmol 0.01 mmol 0.01 mmol |
-
-
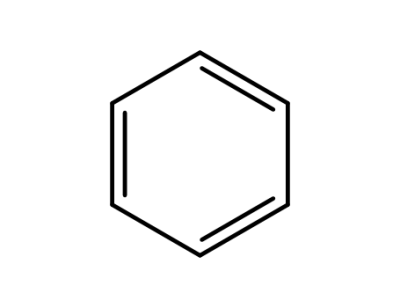
71-43-2,26181-88-4,54682-86-9,13967-78-7,174973-66-1
benzene

-

-
348-51-6
1-chloro-2-fluorobenzene

-
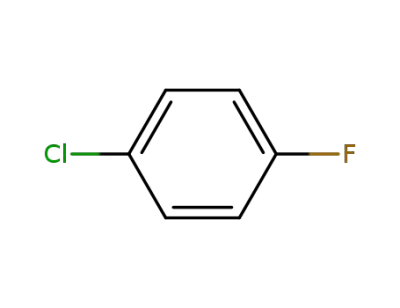
-
352-33-0
1-Chloro-4-fluorobenzene

-
-
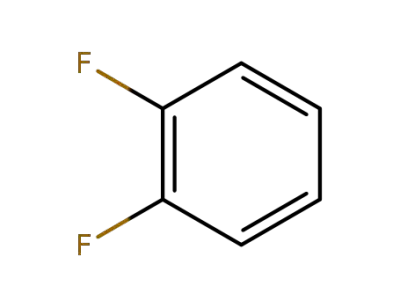
367-11-3
ortho-difluorobenzene

-
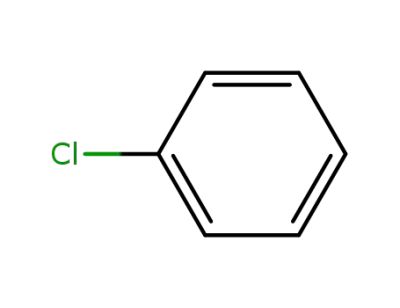
-
108-90-7
chlorobenzene
| Conditions | Yield |
|---|---|
|
With
chlorine pentafluoride;
In
tetrachloromethane;
at 0 ℃;
high ClF5 concentration;
|
5.4% 3.9% 5.2% 12.7% |
352-33-0 Upstream products
-
462-06-6
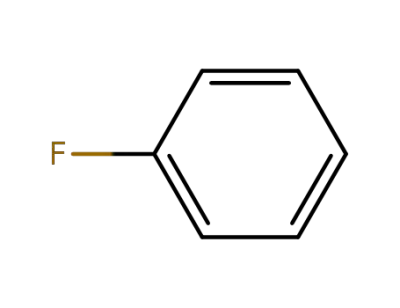
fluorobenzene
-
371-40-4
4-fluoroaniline
-
371-41-5
4-Fluorophenol
-
106-46-7
para-dichlorobenzene
352-33-0 Downstream products
-
88-87-9
4-chloro-2,6-dinitrophenol
-
345-17-5
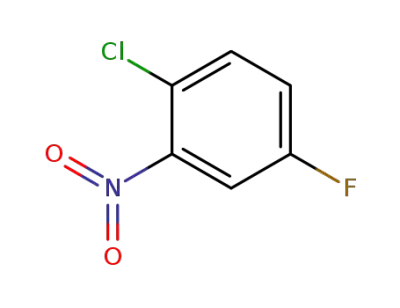
1-chloro-4-fluoro-2-nitrobenzene
-
345-18-6
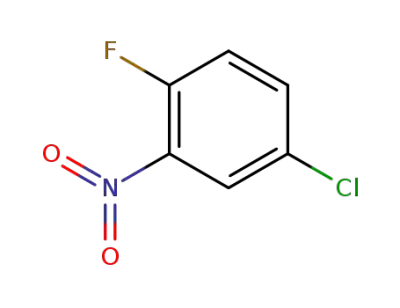
2-fluoro-5-chloronitrobenzene
-
428-15-9
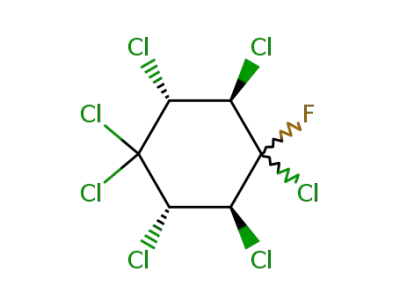
1,1,2r,3t,4ξ,5t,6c-heptachloro-4ξ-fluoro-cyclohexane
Relevant Products
-
1H,1H,2H,2H-Perfluorooctyltrichlorosilane
CAS:78560-45-9
-
2,2'-dithiophene-5,5'-dicarboxaldehyde
CAS:32364-72-0
-
Ethyl 4-(diethoxyphosphoryl)butanoate
CAS:2327-69-7