37500-95-1
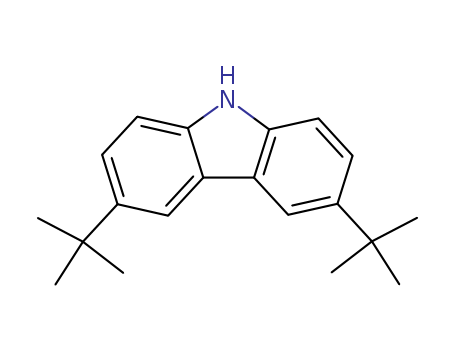
- Product Name:3,6-Di terbutyl carbazole
- Molecular Formula:C20H25 N
- Purity:99%
- Molecular Weight:279.425
Product Details;
CasNo: 37500-95-1
Molecular Formula: C20H25 N
factory and supplier 37500-95-1 3,6-Di terbutyl carbazole in stock
- Molecular Formula:C20H25 N
- Molecular Weight:279.425
- Melting Point:233-235℃
- Boiling Point:424.2±14.0 °C(Predicted)
- PKA:17.70±0.30(Predicted)
- PSA:15.79000
- Density:1.037
- LogP:5.91610
37500-95-1 Relevant articles
Preorganized anion traps for exploiting anion-π interactions: An experimental and computational study
Bretschneider, Anne,Andrada, Diego M.,Dechert, Sebastian,Meyer, Steffen,Mata, Ricardo A.,Meyer, Franc
, p. 16988 - 17000 (2013)
1,3-Bis(pentafluorophenyl-imino)isoindol...
Creation of cationic iridium(iii) complexes with aggregation-induced phosphorescent emission (AIPE) properties by increasing rotation groups on carbazole peripheries
Shan, Guo-Gang,Zhu, Dong-Xia,Li, Hai-Bin,Li, Peng,Su, Zhong-Min,Liao, Yi
, p. 2947 - 2953 (2011)
Three cationic iridium complexes contain...
Carbazole based polymers as hosts for blue iridium emitters: Synthesis, photophysics and high efficiency PLEDs
Stanislovaityte, Egle,Simokaitiene, Jurate,Raisys, Steponas,Al-Attar, Hameed,Grazulevicius, Juozas V.,Monkman, Andrew P.,Jankus, Vygintas
, p. 8209 - 8221 (2013)
This article reports the synthesis of ne...
A route to a cyclobutane-linked double-looped system: Via a helical macrocycle
Klajn, Jan,Stawski, Wojciech,Chmielewski, Piotr J.,Cybińska, Joanna,Pawlicki, Mi?osz
, p. 4558 - 4561 (2019)
Macrocycles built of carbazole and pyrid...
Methoxy- and tert-butyl-substituted meta-bis(N-carbazolyl)phenylenes as hosts for organic light-emitting diodes
Keruckas, Jonas,Volyniuk, Dmytro,Simokaitiene, Jurate,Narbutaitis, Edgaras,Lazauskas, Algirdas,Lee, Pei-Hsi,Chiu, Tien-Lung,Lin, Chi-Feng,Arsenyan, Pavel,Lee, Jiun-Haw,Grazulevicius, Juozas V.
, p. 317 - 326 (2019)
Two new analogues of a popular host mate...
Synthesis and characterization of starburst 9-phenylcarbazole/triazatruxene hybrids
Lai, Wen-Yong,He, Qi-Yuan,Chen, Dao-Yong,Huang, Wei
, p. 986 - 987 (2008)
Novel starburst triazatruxenes functiona...
Thermally activated delayed fluorescence enantiomers for solution-processed circularly polarized electroluminescence
Sun, Sibing,Wang, Jun,Chen, Lingfeng,Chen, Runfeng,Jin, Jibiao,Chen, Cailin,Chen, Shufen,Xie, Guohua,Zheng, Chao,Huang, Wei
, p. 14511 - 14516 (2019)
Circularly polarized organic light-emitt...
Ideal bipolar host materials with bis-benzimidazole unit for highly efficient solution-processed green electrophosphorescent devices
Jiang, Wei,Tang, Jinan,Ban, Xinxin,Sun, Yueming,Duan, Lian,Qiu, Yong
, p. 5346 - 5349 (2014)
An ideal host material with high triplet...
Solution-processible carbazole dendrimers as host materials for highly efficient phosphorescent organic light-emitting diodes
Li, Jiuyan,Zhang, Ting,Liang, Yunjing,Yang, Ruixia
, p. 619 - 628 (2013)
A group of dendrimers with oligo-carbazo...
Carbazole-containing porphyrinoid and its oligomers
Kim, Dongho,Kim, Taeyeon,Song, Jianxin,Wang, Kaisheng,Wu, Tongjing,Xu, Ling,Yin, Bangshao,Zhou, Mingbo
, p. 11454 - 11457 (2019)
A novel carbazole-containing porphyrinoi...
Studies on the Enantioselective Iminium Ion Trapping of Radicals Triggered by an Electron-Relay Mechanism
Bahamonde, Ana,Murphy, John J.,Savarese, Marika,Brémond, éric,Cavalli, Andrea,Melchiorre, Paolo
, p. 4559 - 4567 (2017)
A combination of electrochemical, spectr...
Diphenylaminocarbazoles by 1,8-functionalization of carbazole: Materials and application to phosphorescent organic light-emitting diodes
Ameen, Shahid,Lee, Seul Bee,Yoon, Sung Cheol,Lee, Jaemin,Lee, Changjin
, p. 35 - 44 (2016)
A series of novel carbazole-based materi...
Design, synthesis and application of carbazole macrocycles in anion sensors
Bobacka, Johan,Darnell, Astrid,Haav, Kristjan,Haljasorg, T?iv,Ilisson, Mihkel,Kadam, Sandip A.,Leito, Ivo,Rüütel, Alo,Saar, Indrek,Toom, Lauri,Yrj?n?, Ville
, p. 1901 - 1914 (2020)
Carboxylate sensing solid-contact ion-se...
Carbazolyl-substituted quinazolinones as high-triplet-energy materials for phosphorescent organic light emitting diodes
Gudeika, Dalius,Volyniuk, Dmytro,Mimaite, Viktorija,Lytvyn, Roman,Butkute, Rita,Bezvikonnyi, Oleksandr,Buika, Gintaras,Grazulevicius, Juozas V.
, p. 394 - 405 (2017)
A series of carbazolyl-substituted quina...
Water oxidation catalyzed by a charge-neutral mononuclear ruthenium(III) complex
Lu, Zhongkai,Gao, Yan,Chen, Hu,Liu, Zhao,Sun, Licheng
, p. 1304 - 1310 (2017)
A new charge-neutral Ru(iii) complex RuL...
Synthesis and characterization of D-D-π-A-Type organic dyes bearing carbazole-carbazole as a donor moiety (D-D) for efficient dye-sensitized solar cells
Sudyoadsuk, Taweesak,Pansay, Sakravee,Morada, Somphob,Rattanawan, Rattanawalee,Namuangruk, Supawadee,Kaewin, Tinnagon,Jungsuttiwong, Siriporn,Promarak, Vinich
, p. 5051 - 5063 (2013)
A series of new D-D-π-A-type organic dye...
Optimised synthesis of monoanionic bis(NHC)-pincer ligand precursors and their Li-complexes
Jürgens, Eva,Buys, Kai N.,Schmidt, Anna-Theresa,Furfari, Samantha K.,Cole, Marcus L.,Moser, Michael,Rominger, Frank,Kunz, Doris
, p. 9160 - 9169 (2016)
Herein we report the optimised synthesis...
Deep-blue thermally activated delayed fluorescence dendrimers with?reduced singlet-triplet energy gap for low roll-off non-doped solution-processed organic light-emitting diodes
Li, Jie,Liao, Xiaoqing,Xu, Huixia,Li, Lu,Zhang, Jie,Wang, Hua,Xu, Bingshe
, p. 79 - 86 (2017)
Two TADF dendrimers composed of diphenyl...
Synthesis and photophysical properties of carbazole-based blue light-emitting dendrimers
Adhikari, Ravi M.,Mondal, Rajib,Shah, Bipin K.,Neckers, Douglas C.
, p. 4727 - 4732 (2007)
(Graph Presented) A new class of highly ...
Experimental and theoretical studies on new 7-(3,6-di-tert-butyl-9H-carbazol-9-yl)-10-alkyl-10H-phenothiazine-3-carbaldehydes
Stalindurai, Kesavan,Gokula Krishnan, Kannan,Nagarajan, Erumaipatty Rajagounder,Ramalingan, Chennan
, p. 633 - 643 (2017)
Synthesis of fused heterocyclic aldehyde...
Carbazole dendronised triphenylamines as solution processed high T g amorphous hole-transporting materials for organic electroluminescent devices
Moonsin, Preecha,Prachumrak, Narid,Rattanawan, Ratanawalee,Keawin, Tinnagon,Jungsuttiwong, Siriporn,Sudyoadsuk, Taweesak,Promarak, Vinich
, p. 3382 - 3384 (2012)
Carbazole dendrimers up to 4th generatio...
Strategy to improve the efficiency of solution-processed phosphorescent organic light-emitting devices by modified TADF host with tert-butyl carbazole
Liu, Yan,Qiu, Suyu,Yu, Jianmin,Ban, Xinxin,Pan, Jie,Gao, Kun,Zhu, Aiyun,Zhang, Dongen,Zhang, Tianlin,Tong, Zhiwei
, (2021)
A new bipolar host material t3Cz-SO was ...
Solvatochromic properties of 3,6-di-tert-butyl-8H-indolo[3,2,1-de]acridin- 8-one
Czerwinska, Marlena,Wierzbicka, Malgorzata,Guzow, Katarzyna,Bylinska, Irena,Wiczk, Wieslaw
, p. 19310 - 19320 (2014)
The spectral and photophysical propertie...
Fused heterocycles possessing novel metal-free organic dyes for dye-sensitized solar cells
Stalindurai, Kesavan,Karuppasamy, Ayyanar,Peng, Jia-De,Ho, Kuo-Chuan,Tamilselvan, Annadurai,Ramalingan, Chennan
, p. 278 - 289 (2017)
A series of fused heterocycles possessin...
Polymerizations for olefin-based polymers
-
Page/Page column 48-49, (2022/02/16)
The invention provides a process to form...
High Triplet Energy Iridium(III) Isocyanoborato Complex for Photochemical Upconversion, Photoredox and Energy Transfer Catalysis
Glaser, Felix,Schaer, Raoul,Schmid, Lucius,Wenger, Oliver S.
supporting information, p. 963 - 976 (2022/01/19)
Cyclometalated Ir(III) complexes are oft...
Carbazole-modified thiazolo[3,2-: C] [1,3,5,2]oxadiazaborinines exhibiting aggregation-induced emission and mechanofluorochromism
Potopnyk, Mykhaylo A.,Kravets, Mykola,Luboradzki, Roman,Volyniuk, Dmytro,Sashuk, Volodymyr,Grazulevicius, Juozas Vidas
supporting information, p. 406 - 415 (2021/01/29)
Two highly emissive carbazole-containing...
37500-95-1 Process route
-
-
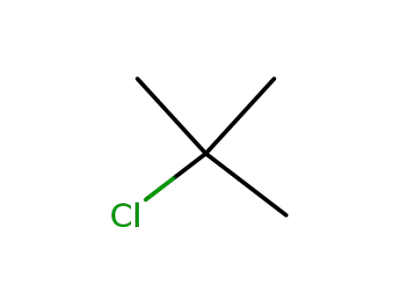
507-20-0
tertiary butyl chloride

-
-
86-74-8,105184-46-1,97960-57-1
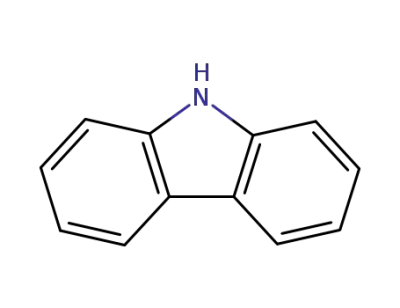
9H-carbazole

-
-
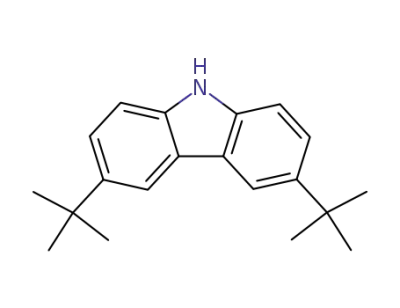
37500-95-1
3,6-di(tert-butyl)-9H-carbazole
| Conditions | Yield |
|---|---|
|
With
zinc(II) chloride;
In
nitromethane;
at 20 ℃;
for 8h;
|
95% |
|
With
zinc(II) chloride;
In
nitromethane;
at 20 ℃;
for 5h;
|
94% |
|
With
zinc(II) chloride;
In
nitromethane;
at 20 ℃;
for 3h;
Inert atmosphere;
Sonication;
|
92% |
|
With
zinc(II) chloride;
In
nitromethane;
for 16h;
Inert atmosphere;
|
86% |
|
With
zinc(II) chloride;
In
nitromethane;
at 20 ℃;
for 5h;
Inert atmosphere;
|
85% |
|
With
zinc(II) chloride;
In
nitromethane;
at 20 ℃;
for 5h;
Inert atmosphere;
|
85% |
|
With
zinc(II) chloride;
In
nitromethane;
at 20 ℃;
for 12h;
Inert atmosphere;
|
85% |
|
With
aluminum (III) chloride;
In
dichloromethane;
at 20 ℃;
for 24h;
|
83% |
|
With
zinc(II) chloride;
In
nitromethane;
at 20 ℃;
for 6h;
Inert atmosphere;
|
81% |
|
With
zinc(II) chloride;
In
nitromethane;
at 20 ℃;
for 5h;
Inert atmosphere;
|
81% |
|
With
aluminum (III) chloride;
In
nitromethane;
at 20 ℃;
Inert atmosphere;
Schlenk technique;
|
79% |
|
With
zinc(II) chloride;
Inert atmosphere;
|
77% |
|
In
nitromethane;
at 20 ℃;
for 8h;
Inert atmosphere;
|
76% |
|
With
nitromethane; zinc(II) chloride;
for 15.33h;
Glovebox;
Inert atmosphere;
|
75% |
|
With
aluminum (III) chloride;
In
dichloromethane;
at 25 ℃;
for 24h;
|
75% |
|
With
zinc(II) chloride;
In
nitromethane;
at 20 ℃;
for 5h;
Inert atmosphere;
|
73% |
|
With
aluminum (III) chloride;
In
dichloromethane;
at 0 - 20 ℃;
for 12h;
Inert atmosphere;
Schlenk technique;
|
70% |
|
With
zinc(II) chloride;
In
nitromethane;
Inert atmosphere;
|
70% |
|
With
aluminum (III) chloride;
In
dichloromethane;
at 0 - 20 ℃;
for 24h;
|
69% |
|
With
aluminum (III) chloride;
In
dichloromethane;
at 20 ℃;
for 16h;
Inert atmosphere;
|
66% |
|
With
aluminum (III) chloride;
In
dichloromethane;
at 0 - 20 ℃;
for 10h;
|
65% |
|
With
aluminum (III) chloride;
In
dichloromethane;
at 20 ℃;
|
61% |
|
With
zinc(II) chloride;
In
nitromethane;
at 20 ℃;
|
60.6% |
|
With
aluminum (III) chloride;
In
dichloromethane;
at 0 - 20 ℃;
for 10.3333h;
Cooling with ice;
|
60% |
|
9H-carbazole;
With
zinc(II) chloride;
In
nitromethane;
at 20 ℃;
for 0.166667h;
tertiary butyl chloride;
In
nitromethane;
for 5h;
|
55% |
|
With
aluminium trichloride;
In
dichloromethane;
at 0 - 20 ℃;
for 24h;
|
54% |
|
With
aluminum (III) chloride;
at 20 ℃;
for 24h;
|
54% |
|
With
aluminum (III) chloride;
In
dichloromethane;
at 0 - 20 ℃;
|
54% |
|
With
aluminum (III) chloride;
In
dichloromethane;
at 0 - 20 ℃;
for 9h;
|
54% |
|
With
aluminum (III) chloride;
In
dichloromethane;
at 0 - 20 ℃;
|
54% |
|
With
aluminum (III) chloride;
In
dichloromethane;
at 0 - 20 ℃;
for 24h;
|
54% |
|
With
aluminum (III) chloride;
In
dichloromethane;
at 0 - 20 ℃;
for 24h;
|
51% |
|
With
aluminum (III) chloride;
|
50% |
|
With
zinc(II) chloride;
In
nitromethane;
at 20 ℃;
for 6h;
Inert atmosphere;
|
50% |
|
With
aluminium trichloride;
In
dichloromethane;
at 20 ℃;
for 16h;
|
47% |
|
With
zinc(II) chloride;
In
nitromethane;
Inert atmosphere;
|
46% |
|
With
zinc(II) chloride;
In
nitromethane;
at 20 ℃;
for 24h;
Inert atmosphere;
|
45% |
|
With
aluminum (III) chloride;
In
chloroform;
at 0 ℃;
for 12h;
|
40% |
|
9H-carbazole;
With
zinc(II) chloride;
In
nitromethane;
at 20 ℃;
Inert atmosphere;
tertiary butyl chloride;
In
nitromethane;
at 20 ℃;
for 5h;
|
40% |
|
With
zinc(II) chloride;
In
nitromethane;
at 20 ℃;
for 5h;
Inert atmosphere;
|
39% |
|
With
aluminum (III) chloride;
In
dichloromethane;
at 20 ℃;
for 48h;
|
37.4% |
|
With
zinc(II) chloride;
In
nitromethane;
at 20 ℃;
for 168h;
Inert atmosphere;
|
36% |
|
With
aluminum (III) chloride;
In
dichloromethane;
at 0 - 20 ℃;
Inert atmosphere;
|
35% |
|
With
zinc(II) chloride;
In
nitromethane;
at 20 ℃;
for 20.5h;
|
32% |
|
With
zinc(II) chloride;
In
nitromethane;
at 20 ℃;
for 20.5h;
Inert atmosphere;
|
32% |
|
With
zinc(II) chloride;
In
nitromethane;
for 5h;
|
11% |
|
With
zinc(II) chloride;
In
nitromethane;
for 5h;
|
11% |
|
With
aluminium trichloride;
|
|
|
With
zinc(II) chloride;
In
nitromethane;
at 40 - 50 ℃;
for 5h;
|
|
|
With
aluminium trichloride;
|
|
|
With
aluminium trichloride;
In
dichloromethane;
|
|
|
With
aluminium trichloride;
|
|
|
With
zinc(II) chloride;
In
nitromethane;
at 40 - 50 ℃;
|
|
|
With
aluminum (III) chloride;
at 20 ℃;
for 24h;
|
|
|
With
zinc(II) chloride;
|
|
|
With
aluminum (III) chloride;
In
dichloromethane;
at 0 - 20 ℃;
Inert atmosphere;
|
|
|
With
zinc(II) chloride;
In
nitromethane;
at 20 ℃;
Molecular sieve;
Inert atmosphere;
|
64.5 mg |
|
With
N-Bromosuccinimide;
|
|
|
With
zinc(II) chloride;
In
nitromethane;
Inert atmosphere;
Schlenk technique;
|
|
|
With
aluminum (III) chloride;
Inert atmosphere;
|
|
|
With
aluminum (III) chloride;
In
dichloromethane;
|
|
|
With
aluminum (III) chloride;
|
|
|
With
aluminum (III) chloride;
In
nitromethane;
at 20 ℃;
for 24h;
|
|
|
With
nitromethane; zinc(II) chloride;
at 20 ℃;
for 5h;
Inert atmosphere;
|
|
|
With
aluminum (III) chloride;
In
dichloromethane;
Inert atmosphere;
|
|
|
With
aluminum (III) chloride;
In
dichloromethane;
at 20 ℃;
for 24h;
|
|
|
With
aluminum (III) chloride;
|
|
|
With
aluminum (III) chloride;
In
dichloromethane;
|
|
|
With
zinc(II) chloride;
In
nitromethane;
at 20 ℃;
for 5h;
|
|
|
With
zinc(II) chloride;
In
nitromethane;
at 20 ℃;
for 5h;
|
|
|
With
aluminum (III) chloride;
|
|
|
With
aluminum (III) chloride;
In
dichloromethane;
at 20 ℃;
for 24h;
|
|
|
With
aluminum (III) chloride;
In
chloroform;
for 16h;
|
|
|
With
aluminum (III) chloride;
In
dichloromethane;
at 0 - 20 ℃;
for 24h;
Inert atmosphere;
|
|
|
With
aluminum (III) chloride;
In
dichloromethane;
at 0 ℃;
for 12h;
|
|
|
With
zinc(II) chloride;
In
nitromethane;
at 20 ℃;
|
|
|
With
zinc(II) chloride;
In
nitromethane;
at 20 ℃;
for 24h;
|
|
|
With
aluminum (III) chloride;
In
dichloromethane;
at 0 - 20 ℃;
Inert atmosphere;
|
9.6 g |
|
With
zinc(II) chloride;
In
nitromethane;
at 20 ℃;
for 24h;
|
|
|
With
zinc(II) chloride;
In
nitromethane; dichloromethane;
for 0.5h;
Sonication;
|
-
-
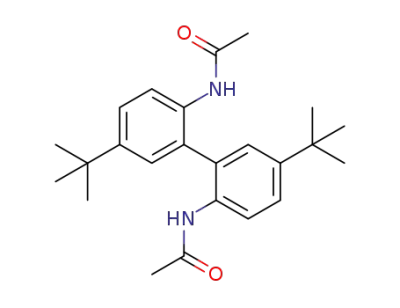
2,2'-bis(acetamido)-5,5'-di-tert-butylbiphenyl

-

-
37500-95-1
3,6-di(tert-butyl)-9H-carbazole
| Conditions | Yield |
|---|---|
|
With
phosphoric acid;
In
diethylene glycol;
at 200 ℃;
for 24h;
|
74% |
37500-95-1 Upstream products
-
507-20-0

tertiary butyl chloride
-
86-74-8

9H-carbazole
-
34601-54-2
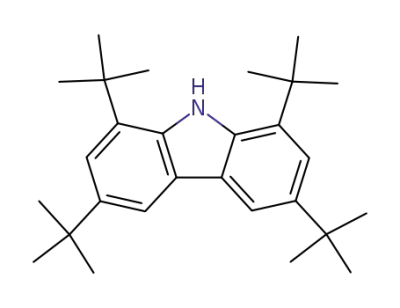
1,3,6,8-tetra-tert-butyl-9H-carbazole
-
7646-85-7
zinc(II) chloride
37500-95-1 Downstream products
-
320575-24-4
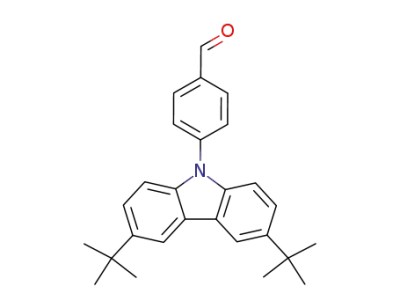
4-(3,6-di-tert-butyl-9H-carbazole-9-yl)benzaldehyde
-
954497-15-5
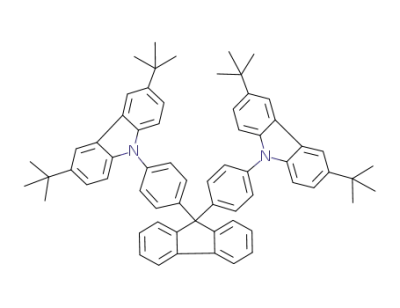
9,9-bis[4-(3,6-di-tert-butyl-9-carbazolyl)phenyl]fluorene
-
1006377-66-7
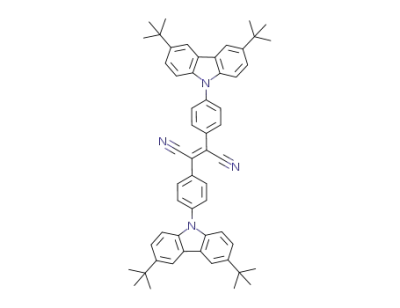
1,2-dicyano-trans-1,2-bis(4-(3,6-di(tert-butyl)carbazol-9-yl)phenyl)ethylene
-
1224836-18-3
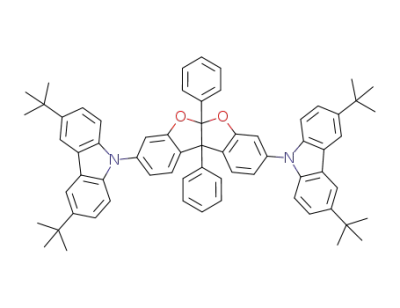
C66H64N2O2
Relevant Products
-
2,5-Dimethoxy-Beta-Nitrostyrene
CAS:40276-11-7
-
1,4-Bis(diphenylphosphino)butane
CAS:7688-25-7
-
5-Bromo-2-fluorotoluene
CAS:51437-00-4