104934-52-3
- Product Name:3-Dodecylthiophene
- Molecular Formula:C16H28S
- Purity:99%
- Molecular Weight:252.46
Product Details;
CasNo: 104934-52-3
Molecular Formula: C16H28S
Appearance: clear colorless to yellow liquid
factory and supplier 104934-52-3 3-Dodecylthiophene in stock
- Molecular Formula:C16H28S
- Molecular Weight:252.46
- Appearance/Colour:clear colorless to yellow liquid
- Vapor Pressure:0.000276mmHg at 25°C
- Melting Point:-0.15°C (estimate)
- Refractive Index:n20/D 1.488(lit.)
- Boiling Point:332.8 °C at 760 mmHg
- Flash Point:114.9 °C
- PSA:28.24000
- Density:0.912 g/cm3
- LogP:6.21150
3-DODECYLTHIOPHENE(Cas 104934-52-3) Usage
|
Preparation |
3-Dodecylthiophene can be prepared from 3-bromothiophene and halogenated hydrocarbons in one step. Steps: Under N2 atmosphere, slowly add 1-bromododecane (28.75g, 26.9 mL) to a 250mL three-necked flask containing a mixture of magnesium chips (3.28g, 0.135mol), anhydrous THF (30mL) and a small amount of iodine. mL, 0.13 mol) in dry THF (45 mL). After the mixture was refluxed at 70 °C for 2 hours, the system was cooled to room temperature with ice water, Ni(dppp)Cl2 (0.54 g, 1.00 mmol) was added first, and then 3-bromothiophene (16.31 g, 0.10 mol) was added slowly. Anhydrous THF (40 mL) solution. The mixed solution was stirred at room temperature overnight, and cold aqueous HCl (1.50 mol/L) was added to quench the reaction. The crude product was extracted with dichloromethane, dried over anhydrous magnesium sulfate, and further purified by column separation purification (n-hexane as eluent), resulting in a clear liquid (22.18 g, yield=88%). |
|
General Description |
3-Dodecylthiophene (3-DT) is a conjugating monomer that can be used as an active layer on semiconductors. It has good electronic properties and can be used in the development of p-type semiconducting polymers. It is mainly used in the formation of poly(3-dodecylthiophene) (P3DT) through electrochemical polymerization. P3DT can further be utilized for a variety of organic electronic based applications. |
InChI:InChI=1/C16H28S/c1-2-3-4-5-6-7-8-9-10-11-12-16-13-14-17-15-16/h13-15H,2-12H2,1H3
104934-52-3 Relevant articles
Synthesis and Structural Characterization of Alkyl Oligothiophenes - The First Isomerically Pure Dialkylsexithiophene
Baeuerle, Peter,Pfau, Frederike,Schlupp, Helge,Wuerthner, Frank,Gaudi, Kai-Uwe,et al.
, p. 489 - 494 (1993)
Regioselective bromination of alkylated ...
Structural Insight into Aggregation and Orientation of TPD-Based Conjugated Polymers for Efficient Charge-Transporting Properties
Lim, Dae-Hee,Kim, Yeon-Ju,Kim, Yeong-A,Hwang, Kyoungtae,Park, Jong-Jin,Kim, Dong-Yu
, p. 4629 - 4638 (2019)
In this study, we obtained a new structu...
Design and synthesis of new ultra-low band gap thiadiazoloquinoxaline-based polymers for near-infrared organic photovoltaic application
Keshtov,Kuklin,Radychev,Nikolaev, A. Yu.,Koukaras,Sharma, Abhishek,Sharma
, p. 14893 - 14908 (2016)
Two D-A copolymers, F1 and F2, with fluo...
Intermolecular Arrangement of Fullerene Acceptors Proximal to Semiconducting Polymers in Mixed Bulk Heterojunctions
Wang, Chao,Nakano, Kyohei,Lee, Hsiao Fang,Chen, Yujiao,Hong, You-Lee,Nishiyama, Yusuke,Tajima, Keisuke
, p. 7034 - 7039 (2018)
Precise control of the molecular arrange...
Optical waveguide fabrication using a polymeric azine containing the 3-dodecyIthiophene moiety
Amari, Claudio,Pelizzi, Corrado,Predieri, Giovanni,Destri, Silvia,Porzio, William,Einsiedel, Heiko,Menges, Bernhard,Mittler-Neher, Silvia
, p. 1319 - 1324 (1996)
New polymeric azines, DOZ and DOPh, cont...
Synthesis of poly(3-dodecyl-2,5-thienylene vinylene) by solid-state metathesis polycondensation
Delgado, Paula A.,Liu, David Y.,Kean, Zachary,Wagener, Kenneth B.
, p. 9529 - 9532 (2011)
The synthesis of poly(thienylene vinylen...
Synthesis and characterization of acceptor-donor-acceptor-based low band gap small molecules containing benzoselenadiazole
Shaik, Baji,Han, Jin-Hee,Song, Dong Jin,Kang, Hun-Min,Lee, Sang-Gyeong
, p. 553 - 558 (2016)
Acceptor-donor-acceptor-type compounds 5...
Synthesis and characterization of a poly(1,3-dithienylisothianaphthene) derivative for bulk heterojunction photovoltaic cells
Vangeneugden,Vanderzande,Salbeck,Van Hal,Janssen,Hummelen,Brabec,Shaheen,Sariciftci
, p. 11106 - 11113 (2001)
The synthesis of a poly(1,3-dithienyliso...
Synthesis and characterization of low-band-gap poly(thienylenevinylene) derivatives for polymer solar cells
Jang, Soo-Young,Lim, Bogyu,Yu, Byung-Kwan,Kim, Juhwan,Baeg, Kang-Jun,Khim, Dongyoon,Kim, Dong-Yu
, p. 11822 - 11830 (2011)
A series of conjugated polymers containi...
Synthesis of donor-acceptor copolymer using benzoselenadiazole as acceptor for OTFT
Shaik, Baji,Han, Jin-Hee,Song, Dong Jin,Kang, Hun-Min,Kim, Ye Beyeol,Park, Chan Eon,Lee, Sang-Gyeong
, p. 4070 - 4076 (2016)
Donor-acceptor-based poly(E)-4-(3,4′-did...
Thiophene-benzothiadiazole based donor–acceptor–donor (D-A-D) bolaamphiphiles, self-assembly and photophysical properties
Chang, Qing,Cheng, Xiaohong,Ding, Wei,Ma, Tao,Zhang, Lin
, (2021/11/03)
Bolaamphiphilies with D-A-D type π-conju...
C(SP3)-C(SP2) CROSS-COUPLING REACTION OF ORGANOZINC REAGENTS AND HETEROCYCLIC (PSEUDO)HALIDES
-
Paragraph 103; 104, (2018/02/28)
Provided is a method of synthesizing a C...
104934-52-3 Process route
-
-
872-31-1
3-Bromothiophene

-

-
143-15-7
1-dodecylbromide

-

-
104934-52-3,104934-53-4
3-dodecylthiophene
| Conditions | Yield |
|---|---|
|
1-dodecylbromide;
With
iodine; magnesium;
In
tetrahydrofuran;
at 70 ℃;
for 2h;
Inert atmosphere;
3-Bromothiophene;
With
1,3-bis[(diphenylphosphino)propane]dichloronickel(II);
In
tetrahydrofuran;
at 20 ℃;
|
88% |
|
1-dodecylbromide;
With
iodine; magnesium;
In
diethyl ether;
for 2h;
Heating;
3-Bromothiophene;
With
nickel dichloride;
In
diethyl ether;
at 20 ℃;
Further stages.;
|
77% |
|
With
1,3-bis[(diphenylphosphino)propane]dichloronickel(II); magnesium;
In
diethyl ether;
|
70% |
|
With
1,3-bis[(diphenylphosphino)propane]dichloronickel(II); magnesium;
In
diethyl ether;
|
70% |
|
1-dodecylbromide;
With
iodine; magnesium;
In
tetrahydrofuran;
at 70 ℃;
for 5h;
Inert atmosphere;
Schlenk technique;
3-Bromothiophene;
With
1,3-bis[(diphenylphosphino)propane]dichloronickel(II);
In
tetrahydrofuran;
Inert atmosphere;
Schlenk technique;
Reflux;
|
60% |
|
With
pyridine; manganese; Tri(p-tolyl)phosphine; cobalt(II) bromide;
In
N,N-dimethyl acetamide;
at 70 ℃;
for 24h;
|
48% |
|
With
1,3-bis[(diphenylphosphino)propane]dichloronickel(II); magnesium;
Yield given. Multistep reaction;
1.) Et2O, reflux, 2 h, 2.) Et2O, reflux, 20 h;
|
|
|
With
1,3-bis[(diphenylphosphino)propane]dichloronickel(II); magnesium;
Yield given. Multistep reaction;
1.) Et2O, 0 deg C, overnight, 2.) Et2O, reflux, 3 h;
|
|
|
With
magnesium;
In
diethyl ether;
|
|
|
3-Bromothiophene; 1-dodecylbromide;
With
magnesium;
In
diethyl ether;
With
1,3-bis[(diphenylphosphino)propane]dichloronickel(II);
|
|
|
With
1,3-bis[(diphenylphosphino)propane]dichloronickel(II); magnesium;
In
tetrahydrofuran;
|
|
|
With
1,3-bis[(diphenylphosphino)propane]dichloronickel(II); magnesium;
In
tetrahydrofuran;
|
-

-
872-31-1
3-Bromothiophene

-

-
869589-06-0
dodecylzinc(II) bromide

-

-
104934-52-3,104934-53-4
3-dodecylthiophene
| Conditions | Yield |
|---|---|
|
With
[1,1'-bis(diphenylphosphino)-ferrocene]palladium(II) chloride-dichlormethane complex;
In
N,N-dimethyl acetamide;
at 80 ℃;
for 12h;
|
95% |
104934-52-3 Upstream products
-
872-31-1

3-Bromothiophene
-
15890-72-9
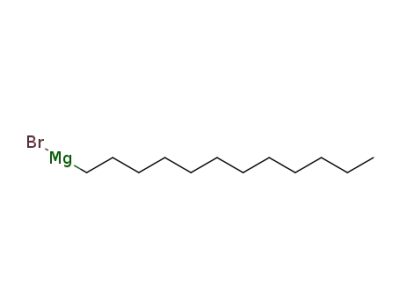
laurylmagnesium bromide
-
143-15-7

1-dodecylbromide
-
905966-46-3
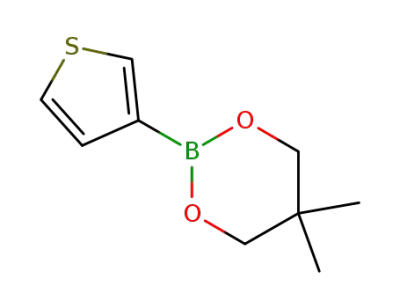
5,5-dimethyl-2-(thiophen-3-yl)-1,3,2-dioxaborinane
104934-52-3 Downstream products
-
350499-09-1
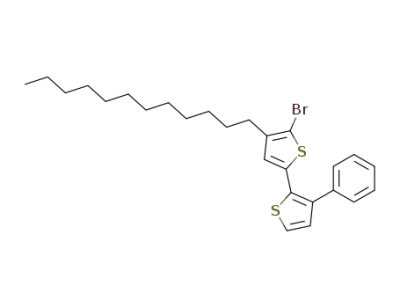
5'-bromo-4'-dodecyl-3-phenyl-[2,2']-bithiophene
-
1170871-42-7
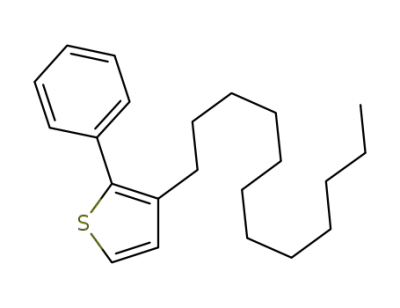
3-dodecyl-2-phenylthiophene
-
1245707-29-2
1,4-bis((E)-2-(5-bromo-3-dodecylthiophen-2-yl)vinyl)benzene
-
1299469-86-5

C23H33BrOS
Relevant Products
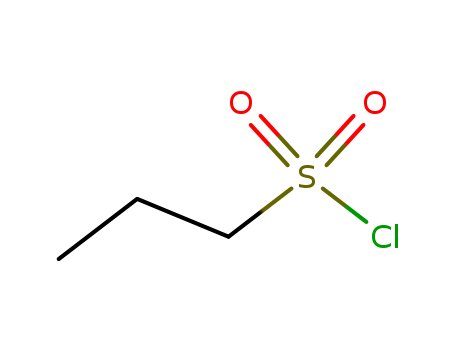
-
Propanesulphonyl chloride
CAS:10147-36-1
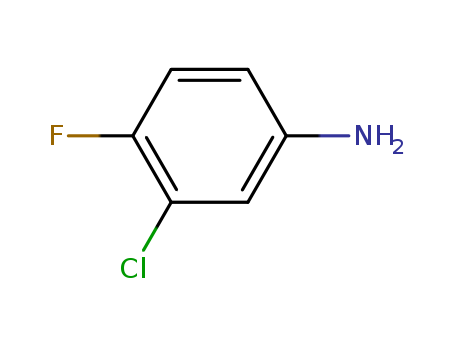
-
3-Chloro-4-fluoroaniline
CAS:367-21-5
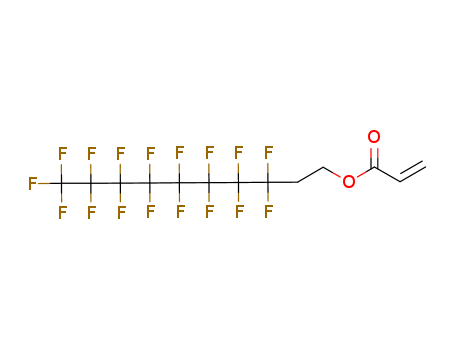
-
Perfluoroalkyl Ethyl Acrylates
CAS:27905-45-9