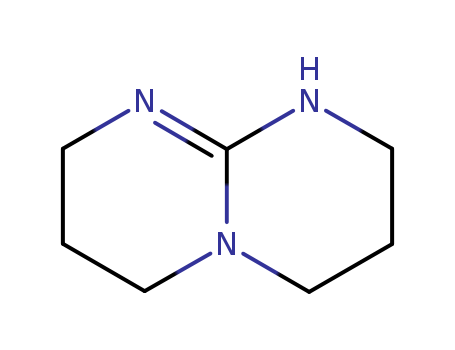
5807-14-7
- Product Name:1,5,7-Triazabicyclo[4.4.0]dec-5-ene
- Molecular Formula:C7H13N3
- Purity:99%
- Molecular Weight:139.2
Product Details;
CasNo: 5807-14-7
Molecular Formula: C7H13N3
Appearance: WHITE TO YELLOW CRYSTALS
factory and supplier 5807-14-7 1,5,7-Triazabicyclo[4.4.0]dec-5-ene in stock
- Molecular Formula:C7H13N3
- Molecular Weight:139.2
- Appearance/Colour:WHITE TO YELLOW CRYSTALS
- Vapor Pressure:0.102mmHg at 25°C
- Melting Point:125-130 °C
- Refractive Index:1.656
- Boiling Point:222.3 °C at 760 mmHg
- PKA:14.47±0.20(Predicted)
- Flash Point:88.3 °C
- PSA:27.63000
- Density:1.28 g/cm3
- LogP:-0.25630
1,3,4,6,7,8-Hexahydro-2H-pyrimido[1,2-a]pyrimidine(Cas 5807-14-7) Usage
|
Purification Methods |
1,5,7-Triazabicyclo[4.4.0]dec-5-ene crystallises from Et2O but readily forms white crystals of the carbonate. It is a strong base (see pK, i.e. about 100 times more basic than tetramethylguanidine). The picrate has m 220.5-222o (from EtOH). It forms the 5-nitro derivative m 14.5-160o that gives a 5-nitro nitrate salt m 100-101o (from EtOH/Et2O) and a 5-nitro picrate m 144-145o (from H2O) [McKay & Kreling Can J Chem 35 1438 1957, Schwesinger Chimia 39 369 1985, Hilpert et al. J Chem Soc, Chem Commun 1401 1983, Kamfen & Eschenmoser Helv Chim Acta 72 185 1989]. [Beilstein 26 III/IV 60.] |
|
General Description |
1,5,7-Triazabicyclo[4.4.0]dec-5-ene, a bicyclic guanidine base, has been found to be an excellent catalyst for Michael and Michael-type reactions. It forms 1:1 complex with lasalocid acid and crystal structure of the complex has been studied by X-ray diffraction, FT-IR spectroscopy and 1H NMR. |
InChI:InChI=1/C7H13N3/c1-3-8-7-9-4-2-6-10(7)5-1/h1-6H2,(H,8,9)
5807-14-7 Relevant articles
Super alkali material and preparation method thereof, and organic light-emitting diode
-
Paragraph 0056-0060, (2022/03/18)
The invention discloses a super alkali m...
Synthesis method of 7-methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene
-
Paragraph 0019-0021; 0029-0031, (2021/08/11)
The invention discloses a synthesis meth...
PROCESS FOR PREPARING BICYCLIC GUANIDINES
-
Paragraph 0103-0109, (2021/07/17)
Bicyclic guanidines are prepared by reac...
PROCESS FOR THE PRODUCTION OF CYCLIC GUANIDINE DERIVATES
-
Page/Page column 12-13, (2021/04/30)
The present invention relates to a proce...
5807-14-7 Process route
-
-
74569-04-3
3,4,6,7,8,9-Hexahydro-2H-pyrimido<1,2-a>pyrimidin-hydrobromid

-
![1,3,4,6,7,8-hexahydro-2H-pyrimido[1,2-a]pyrimidine](/upload/2025/12/a4708755-9c6c-4c15-b699-811494f06b63.png)
-
5807-14-7,389623-48-7
1,3,4,6,7,8-hexahydro-2H-pyrimido[1,2-a]pyrimidine
| Conditions | Yield |
|---|---|
|
With
sodium methylate;
In
ethanol;
at 0 - 20 ℃;
for 24h;
Inert atmosphere;
Large scale;
|
95% |
-
-
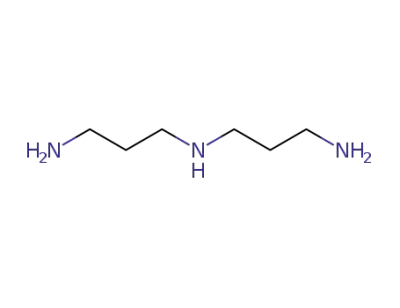
56-18-8
bis(3-aminopropyl)amine

-
-
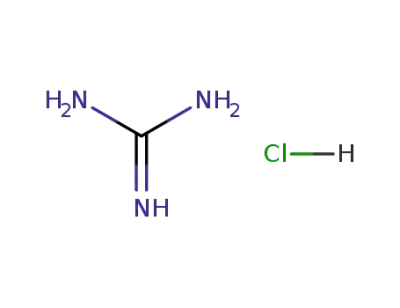
50-01-1
guanidine hydrochloride

-
![1,3,4,6,7,8-hexahydro-2H-pyrimido[1,2-a]pyrimidine](/upload/2025/12/a4708755-9c6c-4c15-b699-811494f06b63.png)
-
5807-14-7,389623-48-7
1,3,4,6,7,8-hexahydro-2H-pyrimido[1,2-a]pyrimidine
| Conditions | Yield |
|---|---|
|
bis(3-aminopropyl)amine; guanidine hydrochloride;
With
hydrogenchloride;
at 155 ℃;
With
sodium methylate;
In
methanol;
|
93% |
|
With
anion exchange resin (D201);
In
water; 1,3,5-trimethyl-benzene;
at 120 ℃;
for 9h;
Temperature;
Autoclave;
Inert atmosphere;
|
55% |
5807-14-7 Upstream products
-
229311-99-3
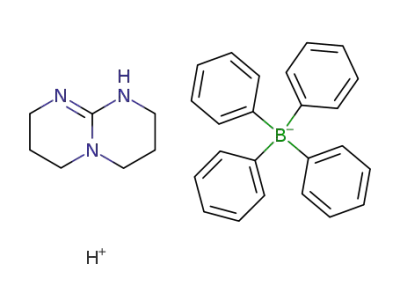
1,3,4,6,7,8-hexahydro-2H-pyrimido[1,2-a]pyrimidine tetraphenylborate
-
13173-07-4
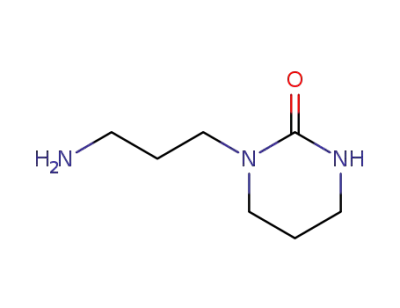
1-(3-aminopropyl)tetrahydro-2(1H)-pyrimidinone
-
109-73-9
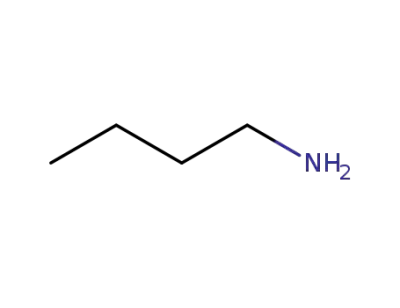
N-butylamine
-
100-51-6
benzyl alcohol
Relevant Products
-
2,5-Dimethoxy-Beta-Nitrostyrene
CAS:40276-11-7
-
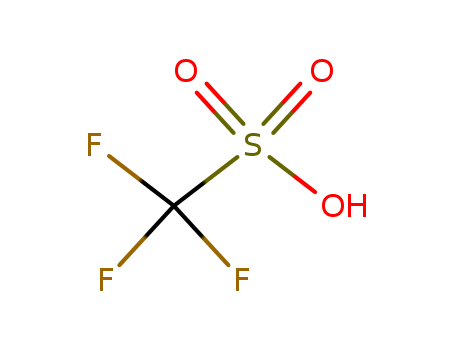
Trifluoromethanesulfonic acid
CAS:1493-13-6
-
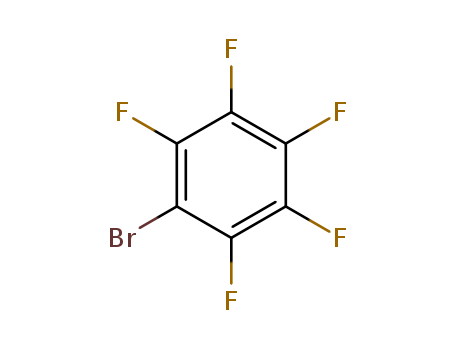
Bromopentafluorobenzene
CAS:344-04-7