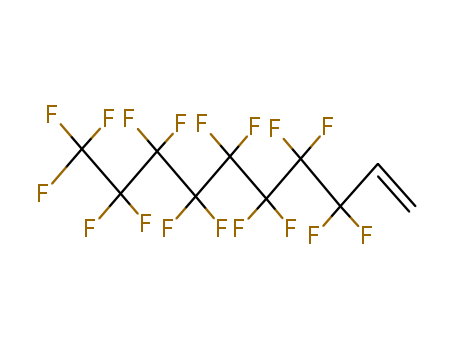2270-59-9
- Product Name:5-Bromo-2-methylpent-2-ene
- Molecular Formula:C6H11Br
- Purity:99%
- Molecular Weight:163.057
Product Details;
CasNo: 2270-59-9
Molecular Formula: C6H11Br
Appearance: Colorless Liquid
factory and supplier 2270-59-9 5-Bromo-2-methylpent-2-ene in stock
- Molecular Formula:C6H11Br
- Molecular Weight:163.057
- Appearance/Colour:Colorless Liquid
- Vapor Pressure:4.44mmHg at 25°C
- Melting Point:-102.35°C (estimate)
- Refractive Index:n20/D 1.476(lit.)
- Boiling Point:152.6 °C at 760 mmHg
- Flash Point:22.8 °C
- PSA:0.00000
- Density:1.215 g/cm3
- LogP:2.73760
5-BROMO-2-METHYL-2-PENTENE(Cas 2270-59-9) Usage
InChI:InChI=1/C6H11Br/c1-6(2)4-3-5-7/h4H,3,5H2,1-2H3
2270-59-9 Relevant articles
-
Buchbauer,G.
, p. 289 - 302 (1978)
-
Studies in Cephalotaxus Alkaloids. Stereospecific Total Synthesis of Homoharringtonine
Hiranuma, Sayoko,Shibata, Misako,Hudlicky, Tomas
, p. 5321 - 5326 (1983)
The alkaloid ester homoharringtonine (2)...
Synthesis of homoharringtonine and its derivative by patial esterification of cephalotaxine
Hiranuma,Hudlicky
, p. 3431 - 3434 (1982)
-
Total synthesis and biological evaluation of the natural product (-)-cyclonerodiol, a new inhibitor of IL-4 signaling
Langhanki, Jens,Rudolph, Kristina,Erkel, Gerhard,Opatz, Till
, p. 9707 - 9715 (2014)
In a screening program of natural compou...
Progress toward the total synthesis of bacchopetiolone: Application of a tandem aromatic oxidation/Diels-Alder reaction
Berube, Amelie,Drutu, Ioana,Wood, John L.
, p. 5421 - 5424 (2006)
A stereoselective synthesis of the bacch...
-
Slobodin et al.
, p. 1873,1876,1877; engl.Ausg.S.1979,1980,1981 (1953)
-
Towards the total synthesis of vibsanin E, 15-O-methylcyclovibsanin B, 3-hydroxyvibsanin E, furanovibsanin A, and 3-O-methylfuranovibsanin A
Schwartz, Brett D.,Tilly, David P.,Heim, Ralf,Wiedemann, Stefan,Williams, Craig M.,Bernhardt, Paul V.
, p. 3181 - 3192 (2006)
Studies detailing synthetic approaches t...
Photoredox-Catalyzed Cascade Reactions Involving Aryl Radical: Total Synthesis of (±)-Norascyronone A and (±)-Eudesmol
Xu, Ze-Jun,Liu, Xu-Yuan,Zhu, Ming-Zhu,Xu, Yu-Liang,Yu, Yue,Xu, Hai-Ruo,Cheng, Ai-Xia,Lou, Hong-Xiang
supporting information, p. 9073 - 9077 (2021/12/06)
Herein, we have developed two types of p...
Incorporation of a FRET pair within a phosphonate diester
Harmon, Nyema M.,Huang, Xueting,Hsiao, Chia-Hung Christine,Wiemer, Andrew J.,Wiemer, David F.
, (2021/06/16)
Cell-cleavable protecting groups are an ...
Direct Synthesis of Enones by Visible-Light-Promoted Oxygenation of Trisubstituted Olefins Using Molecular Oxygen
Harada, Shinji,Matsuda, Daiki,Morikawa, Takahiro,Nishida, Atsushi
supporting information, p. 1372 - 1377 (2020/10/02)
A one-step synthesis of enones from olef...
AuCl3-Catalyzed Ring-Closing Carbonyl–Olefin Metathesis
Wang, Rui,Chen, Yi,Shu, Mao,Zhao, Wenwen,Tao, Maoling,Du, Chao,Fu, Xiaoya,Li, Ao,Lin, Zhihua
supporting information, p. 1941 - 1946 (2020/02/11)
Compared with the ripeness of olefin met...
2270-59-9 Process route
-
-
765-43-5
Cyclopropyl methyl ketone

-
-
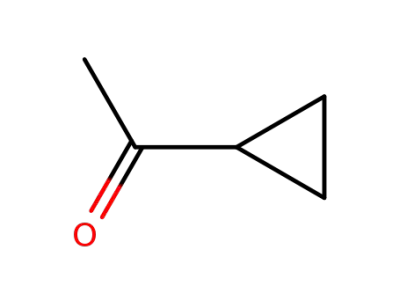
75-16-1
methylmagnesium bromide

-

-
2270-59-9
1-bromo-4-methylpent-3-ene
| Conditions | Yield |
|---|---|
|
Cyclopropyl methyl ketone; methylmagnesium bromide;
In
tetrahydrofuran;
for 1h;
Reflux;
Inert atmosphere;
With
sulfuric acid;
In
tetrahydrofuran; water;
at 10 ℃;
for 0.5h;
Inert atmosphere;
|
88% |
|
Cyclopropyl methyl ketone; methylmagnesium bromide;
In
tetrahydrofuran;
at 20 ℃;
Inert atmosphere;
Reflux;
With
sulfuric acid;
In
tetrahydrofuran;
at 10 ℃;
|
85% |
|
With
sulfuric acid;
In
diethyl ether;
for 1h;
|
85% |
|
Cyclopropyl methyl ketone; methylmagnesium bromide;
With
sulfuric acid;
|
80% |
|
Cyclopropyl methyl ketone; methylmagnesium bromide;
In
tetrahydrofuran; diethyl ether;
for 0.833333h;
With
sulfuric acid; water;
In
tetrahydrofuran; diethyl ether;
for 0.5h;
|
77% |
|
Cyclopropyl methyl ketone; methylmagnesium bromide;
In
tetrahydrofuran;
at 30 - 35 ℃;
for 1h;
With
sulfuric acid;
In
tetrahydrofuran; water;
at 0 - 30 ℃;
for 0.25h;
|
77% |
|
Cyclopropyl methyl ketone; methylmagnesium bromide;
In
tetrahydrofuran;
at 30 - 35 ℃;
for 1h;
With
sulfuric acid;
In
tetrahydrofuran; water;
at 0 - 30 ℃;
for 0.25h;
|
77% |
|
Cyclopropyl methyl ketone; methylmagnesium bromide;
In
tetrahydrofuran; diethyl ether;
for 0.333333h;
Inert atmosphere;
Reflux;
With
sulfuric acid;
In
tetrahydrofuran; diethyl ether; water;
for 0.5h;
Inert atmosphere;
|
77% |
|
In
tetrahydrofuran; diethyl ether;
for 0.5h;
Reflux;
|
73% |
|
Cyclopropyl methyl ketone; methylmagnesium bromide;
In
tetrahydrofuran;
for 0.333333h;
Reflux;
Inert atmosphere;
With
sulfuric acid;
In
water;
at 10 ℃;
for 0.5h;
Inert atmosphere;
|
71% |
|
Cyclopropyl methyl ketone; methylmagnesium bromide;
In
tetrahydrofuran; diethyl ether;
at 0 ℃;
Reflux;
With
sulfuric acid;
In
tetrahydrofuran; diethyl ether; water;
at 0 - 20 ℃;
|
70% |
|
Cyclopropyl methyl ketone; methylmagnesium bromide;
In
tetrahydrofuran;
at 0 - 40 ℃;
for 2h;
With
sulfuric acid;
In
water;
at 0 ℃;
for 1h;
|
53% |
|
In
tetrahydrofuran;
at 0 ℃;
for 0.333333h;
Reflux;
|
33% |
|
Multistep reaction;
(i) Et2O, (ii) aq. HBr;
|
|
|
Cyclopropyl methyl ketone; methylmagnesium bromide;
In
tetrahydrofuran;
at 0 ℃;
for 0.5h;
Inert atmosphere;
Reflux;
With
sulfuric acid;
In
tetrahydrofuran; water;
at 0 - 20 ℃;
for 1h;
Inert atmosphere;
|
|
|
Cyclopropyl methyl ketone; methylmagnesium bromide;
In
tetrahydrofuran;
for 1h;
Inert atmosphere;
Reflux;
With
sulfuric acid;
In
tetrahydrofuran; water;
at 0 ℃;
for 0.5h;
Inert atmosphere;
|
|
|
Cyclopropyl methyl ketone; methylmagnesium bromide;
In
diethyl ether;
at 0 ℃;
With
sulfuric acid;
In
diethyl ether;
at 0 ℃;
|
|
|
Cyclopropyl methyl ketone; methylmagnesium bromide;
In
diethyl ether;
Inert atmosphere;
With
sulfuric acid;
Inert atmosphere;
|
-
-
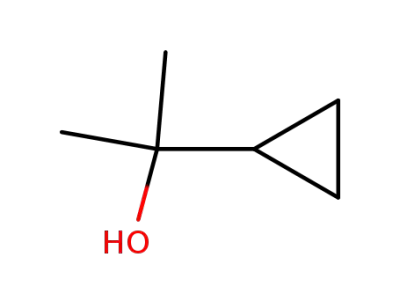
930-39-2
2-cyclopropyl-2-propanol

-

-
2270-59-9
1-bromo-4-methylpent-3-ene
| Conditions | Yield |
|---|---|
|
With
magnesium bromide;
In
diethyl ether;
for 3h;
Heating;
|
93% |
|
With
magnesium bromide;
In
diethyl ether;
for 8h;
Heating;
|
89% |
|
With
hydrogen bromide; lithium bromide;
In
water;
at 0 ℃;
for 0.75h;
|
87% |
|
With
hydrogen bromide;
In
diethyl ether;
|
83% |
|
With
hydrogen bromide;
In
Petroleum ether;
for 0.166667h;
|
63% |
|
With
pyridine; diethyl ether; phosphorus tribromide;
|
|
|
With
water; hydrogen bromide;
|
|
|
With
hydrogen bromide;
|
|
|
With
hydrogen bromide;
at 5 ℃;
|
|
|
With
hydrogen bromide;
In
pentane;
|
|
|
With
hydrogen bromide;
|
|
|
With
magnesium bromide;
In
diethyl ether;
for 8h;
Heating;
|
100 % Chromat. |
|
With
hydrogen bromide;
for 0.5h;
ice bath;
|
|
|
With
hydrogen bromide;
|
|
|
With
hydrogen bromide;
for 1h;
Yield given;
|
|
|
With
hydrogen bromide;
In
water;
at 20 ℃;
for 2.333h;
|
|
|
With
hydrogen bromide;
In
water;
at 20 ℃;
for 2h;
Inert atmosphere;
|
2270-59-9 Upstream products
-
52278-94-1
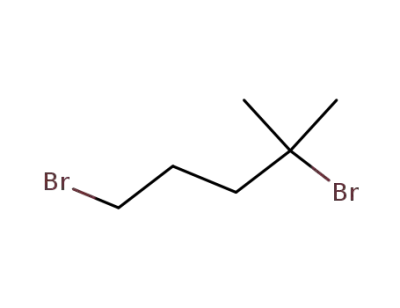
2,5-dibromo-2-methylpentane
-
763-89-3
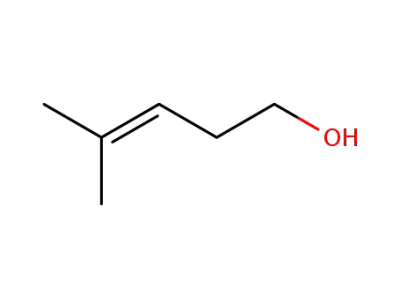
4-methylpent-3-en-1-ol
-
930-39-2

2-cyclopropyl-2-propanol
-
582-25-2
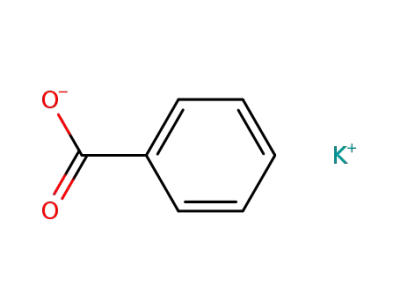
Potassium benzoate
2270-59-9 Downstream products
-
78-70-6
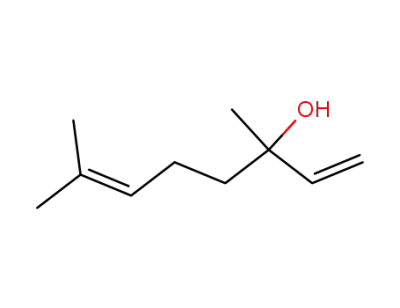
3,7-dimethylocta-1,6-dien-3-ol
-
926-56-7
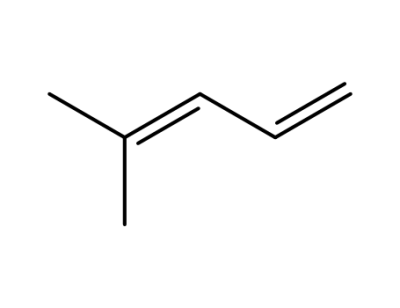
4-methyl-1,3-pentadiene
-
42272-94-6
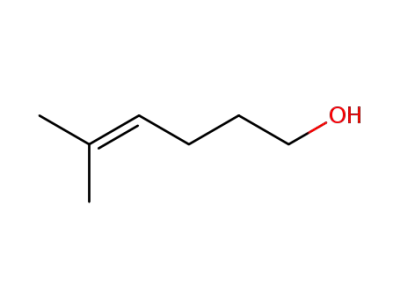
5-methylhex-4-ene-1-ol
-
22410-72-6
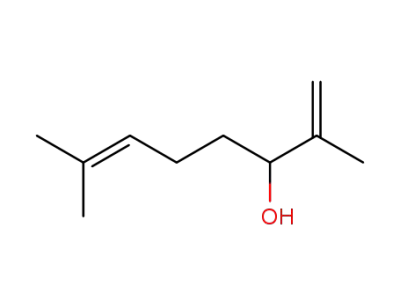
(+/-)-lavandulol
Relevant Products
-
5-Bromo-2-fluoro-m-xylene
CAS:99725-44-7
-
2-Perfluorooctyl Ethylene
CAS:21652-58-4
-
4-Tritylaniline
CAS:22948-06-7