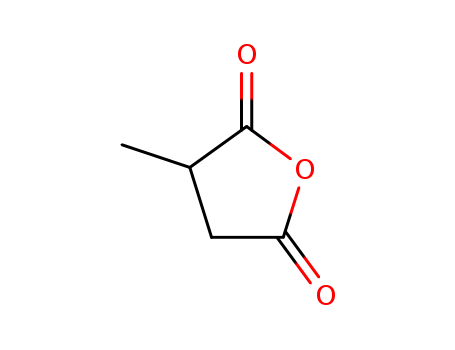
4100-80-5
- Product Name:Methylsuccinic Anhydride
- Molecular Formula:C5H6O3
- Purity:99%
- Molecular Weight:114.101
Product Details;
CasNo: 4100-80-5
Molecular Formula: C5H6O3
factory and supplier 4100-80-5 Methylsuccinic Anhydride in stock
- Molecular Formula:C5H6O3
- Molecular Weight:114.101
- Vapor Pressure:0.0411mmHg at 25°C
- Melting Point:33-35 °C(lit.)
- Refractive Index:1.452
- Boiling Point:239 °C at 760 mmHg
- Flash Point:108.2 °C
- PSA:43.37000
- Density:1.23 g/cm3
- LogP:0.09600
METHYLSUCCINIC ANHYDRIDE(Cas 4100-80-5) Usage
|
Definition |
ChEBI: A tetrahydrofurandione that is succinic anhydride substituted by a methyl group at position 3. |
InChI:InChI=1/C5H6O3/c1-3-2-4(6)8-5(3)7/h3H,2H2,1H3/t3-/m0/s1
4100-80-5 Relevant articles
-
Bergmann,Blum-Bergmann
, p. 1573 (1937)
-
Carbonylative Polymerization of Epoxides Mediated by Tri-metallic Complexes: A Dual Catalysis Strategy for Synthesis of Biodegradable Polyhydroxyalkanoates
Li, Wen-Bing,Liu, Ye,Lu, Xiao-Bing,Yang, Jin-Chuang,Yang, Jun
supporting information, (2022/01/20)
Polyhydroxyalkanoates (PHAs) are a uniqu...
Synthesis of Cyclic Anhydrides via Ligand-Enabled C–H Carbonylation of Simple Aliphatic Acids
Herron, Alastair N.,Yu, Jin-Quan,Zhuang, Zhe
supporting information, p. 16382 - 16387 (2021/06/23)
The development of C(sp3)–H functionaliz...
Thiol–Anhydride Dynamic Reversible Networks
Podgórski, Maciej,Mavila, Sudheendran,Huang, Sijia,Spurgin, Nathan,Sinha, Jasmine,Bowman, Christopher N.
supporting information, p. 9345 - 9349 (2020/04/07)
The reaction of thiols and anhydrides to...
(Meth) acrylic ester and manufacturing method thereof (by machine translation)
-
Paragraph 0058, (2019/12/04)
(Meth) acrylic acid ester of [be] hydrop...
4100-80-5 Process route
-
-
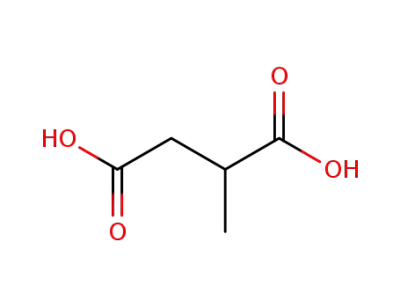
498-21-5,636-60-2
2-methylbutanedioic acid

-
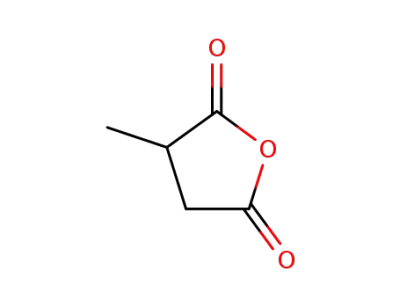
-
4100-80-5
2-methylsuccinic anhydride
| Conditions | Yield |
|---|---|
|
With
oxalyl dichloride; N,N-dimethyl-formamide;
In
toluene;
for 3h;
Inert atmosphere;
Reflux;
|
96% |
|
With
acetic anhydride;
for 4h;
Reflux;
|
88.2% |
|
With
acetyl chloride;
|
|
|
at 200 ℃;
inactive form;
|
|
|
With
diphosphorus pentasulfide;
inactive form;
|
|
|
With
acetyl chloride;
inactive form;
|
|
|
With
acetic anhydride;
|
|
|
With
oxalyl dichloride;
for 0.333333h;
Heating;
|
|
|
With
4-methyl-morpholine; methyl chloroformate;
In
tetrahydrofuran;
at 20 ℃;
for 0.25h;
|
|
|
at 66.9 ℃;
Equilibrium constant;
Thermodynamic data;
-ΔΔH;
|
|
|
With
acetyl chloride;
|
-
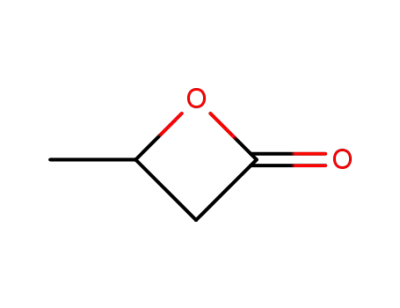
-
3068-88-0,32082-74-9,36536-46-6,65058-82-4
4-methyloxetan-2-one

-
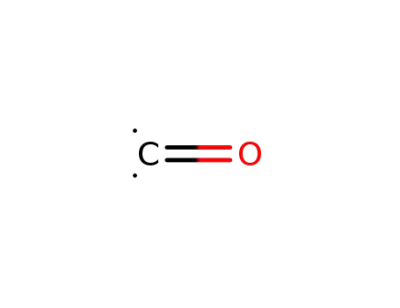
-
201230-82-2
carbon monoxide

-

-
4100-80-5
2-methylsuccinic anhydride
| Conditions | Yield |
|---|---|
|
With
({1,2-di[(2-OH-3,5-di-tBu)PhCH=N]Ph}Al[THF]2)+[Co(CO)4]-;
In
toluene;
at 55 ℃;
for 24h;
under 10343 Torr;
|
95% |
|
[(meso-tetra(4-Cl-C6H4)porphyrinato)Al(THF)2]+[Co(CO)4]-;
In
1,4-dioxane;
at 40 ℃;
under 43958.7 Torr;
Further Variations:;
Solvents;
Pressures;
Kinetics;
|
|
|
With
Co(CO)4?Cr-MIL-101 MOF; silicon carbide powder;
In
toluene;
at 40 ℃;
for 60h;
under 22502.3 Torr;
Pressure;
Temperature;
chemoselective reaction;
Catalytic behavior;
Mechanism;
Inert atmosphere;
Glovebox;
Flow reactor;
|
4100-80-5 Upstream products
-
498-21-5

2-methylbutanedioic acid
-
616-02-4
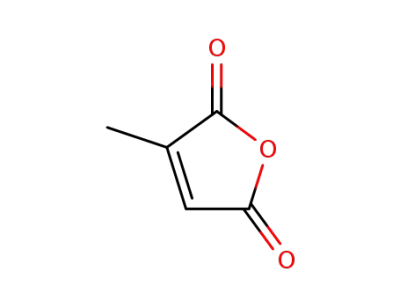
citraconic acid anhydride
-
10458-14-7
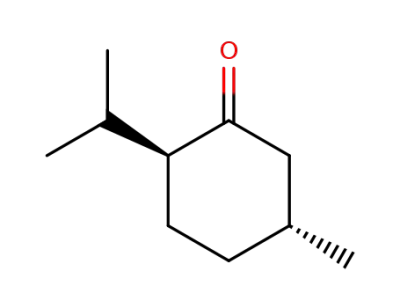
(2R,5S)-menthone
-
2170-03-8
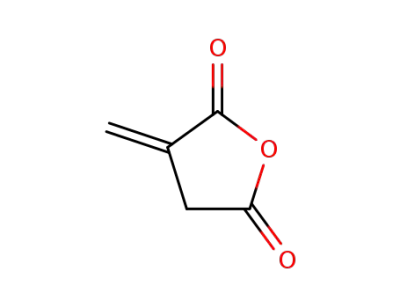
itaconic acid anhydride
4100-80-5 Downstream products
-
4676-51-1
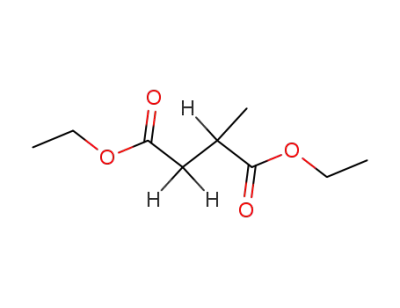
Diethyl 2-methylsuccinate
-
105475-81-8
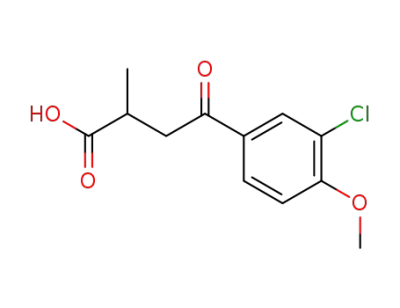
4-(3-chloro-4-methoxy-phenyl)-2-methyl-4-oxo-butyric acid
-
102706-93-4
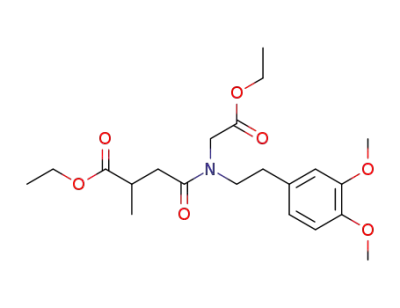
N-(3-ethoxycarbonyl-butyryl)-N-(3,4-dimethoxy-phenethyl)-glycine ethyl ester
-
105475-32-9
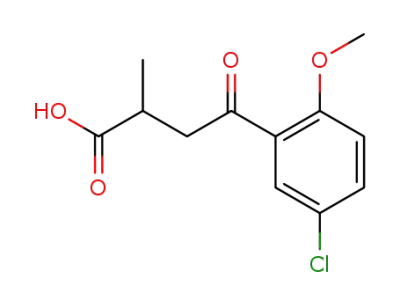
4-(5-chloro-2-methoxy-phenyl)-2-methyl-4-oxo-butyric acid
Relevant Products
-
DIBASIC ESTER
CAS:95481-62-2
-
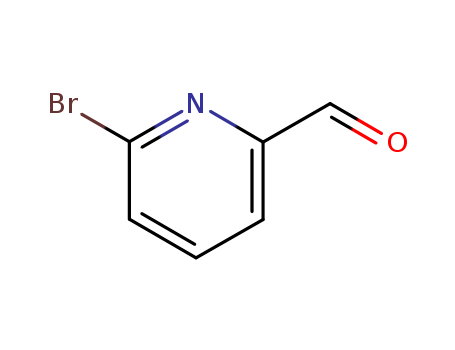
6-Bromopyridine-2-carbaldehyde
CAS:34160-40-2
-
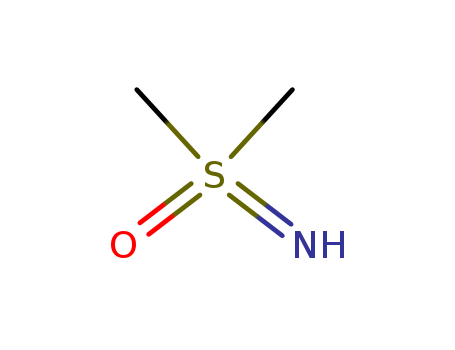
S,S-dimethyl sulfoximine
CAS:1520-31-6