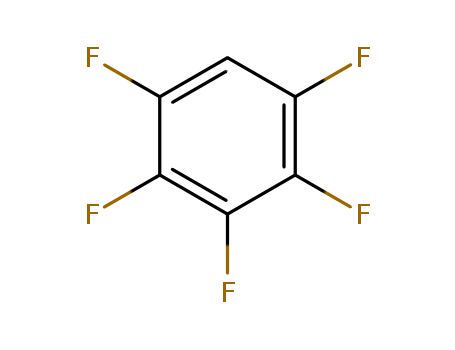
363-72-4
- Product Name:Pentafluorobenzene
- Molecular Formula:C6HF5
- Purity:99%
- Molecular Weight:168.066
Product Details;
CasNo: 363-72-4
Molecular Formula: C6HF5
Appearance: clear colorless to light yellow liquid
factory and supplier 363-72-4 Pentafluorobenzene in stock
- Molecular Formula:C6HF5
- Molecular Weight:168.066
- Appearance/Colour:clear colorless to light yellow liquid
- Vapor Pressure:77.3mmHg at 25°C
- Melting Point:-48 °C(lit.)
- Refractive Index:n20/D 1.391(lit.)
- Boiling Point:85.5 °C at 760 mmHg
- Flash Point:13.9 °C
- PSA:0.00000
- Density:1.521 g/cm3
- LogP:2.38210
Pentafluorobenzene(Cas 363-72-4) Usage
|
Purification Methods |
Purify it by distillation and by gas chromatography. IR film: 1535 and 1512 cm-1 (*C6H6 ring). [Stephen & Tatlow Chem Ind (London) 821 1957, Nield et al. J Chem Soc 166 1959, Beilstein 5 IV 639.] |
|
General Description |
Pentafluorobenzene is a fluorinated aromatic compound that serves as a key intermediate in nucleophilic substitution reactions, particularly in the formation of perfluorinated indane derivatives through intramolecular cyclization. Its reactivity is exemplified in reactions with perfluoroolefins, where it can generate perfluorinated carbanions that further cyclize or form arylolefins, demonstrating its versatility in synthesizing complex fluorinated structures. |
InChI:InChI=1/C6HF5/c7-2-1-3(8)5(10)6(11)4(2)9/h1H
363-72-4 Relevant articles
Heterolytic bond activation at gold: Evidence for gold(iii) H-B, H-Si complexes, H-H and H-C cleavage
Rocchigiani, Luca,Budzelaar, Peter H. M.,Bochmann, Manfred
, p. 2633 - 2642 (2019)
The coordinatively unsaturated gold(iii)...
The fluorine-pentafluorophenyl substitution reaction in anhydrous hydrogen fluoride (aHF): A new interesting methodical approach to synthesize pentafluorophenylxenonium salts
Frohn, Hermann-Josef,Schroer, Thorsten
, p. 259 - 264 (2001)
In anhydrous hydrogen fluoride (aHF) (he...
Synthesis and Reactivity of a Low-Coordinate Iron(II) Hydride Complex: Applications in Catalytic Hydrodefluorination
Hein, Nicholas M.,Pick, Fraser S.,Fryzuk, Michael D.
, p. 14513 - 14523 (2017)
A low-coordinate iron hydride complex be...
Promotion of reductive elimination reaction of diorgano(2,2′-bipyridyl)nickel(II) complexes by electron-accepting aromatic compounds, Lewis acids, and Bronsted acids
Yamamoto, Takakazu,Abla, Mahmut,Murakami, Yasuharu
, p. 1997 - 2009 (2002)
Reductive elimination of R-R from dialky...
Promoting Difficult Carbon–Carbon Couplings: Which Ligand Does Best?
Gioria, Estefanía,del Pozo, Juan,Martínez-Ilarduya, Jesús M.,Espinet, Pablo
, p. 13276 - 13280 (2016)
A Pd complex, cis-[Pd(C6F5)2(THF)2] (1),...
The Pentafluorophenylxenon(II) Cation: +; The First Stable System with a Xenon-Carbon Bond
Frohn, Hermann J.,Jakobs, Stephanus
, p. 625 - 627 (1989)
Pentafluorophenylxenon(II) pentafluoroph...
Reaction of difluoromethyl pentafluorophenyl sulfoxide with nucleophiles
Koshcheev,Maksimov,Platonov,Shelkovnikov
, (2017)
Reactions of 1-(difluoromethanesulfinyl)...
-
Klabunde et al.
, p. 207 (1972)
-
Grignard exchange reaction using a microflow system: From bench to pilot plant
Wakami, Hideo,Yoshida, Jun-Ichi
, p. 787 - 791 (2005)
The Grignard exchange reaction of ethylm...
Reductive elimination of C6F5-C6F 5 in the reaction of bis(pentafluorophenyl)palladium(ii) complexes with protic acids
Koizumi, Take-Aki,Yamazaki, Atsuko,Yamamoto, Takakazu
, p. 3949 - 3952 (2008)
Reductive elimination of C6F5-C6F 5 from...
Synthesis, reactivity and X-ray crystal structure of tris(pentafluorophenyl)silanol (C6F5)3SiOH
Cariati, Elena,Carlucci, Lucia,D'Alfonso, Giuseppe,Giovenzana, Tommaso,Lucenti, Elena,Maggioni, Daniela,Sironi, Angelo
, (2022/01/26)
Tris(pentafluorophenyl)silanol (C6F5)3Si...
Convenient Synthesis of Symmetrical Polyfluorinated Diphenyl Sulfides
Bredikhin, R. A.,Maksimov, A. M.,Nikul’shin, P. V.,Platonov, V. E.
, p. 1921 - 1930 (2022/01/24)
Abstract: Thermal properties of decafluo...
Protodeboronation of (Hetero)Arylboronic Esters: Direct versus Prehydrolytic Pathways and Self-/Auto-Catalysis
Assante, Michele,Geogheghan, Katherine J.,Hayes, Hannah L. D.,Jin, Na,Leach, Andrew G.,Lloyd-Jones, Guy C.,Noonan, Gary,Tomasi, Simone,Wei, Ran
supporting information, p. 14814 - 14826 (2021/09/13)
The kinetics and mechanism of the base-c...
The aliphatic ring-opening and SNAr substitution in the reactions of perfluorobenzocycloalkenones with K2CO3 in water and methanol
Zonov, Yaroslav V.,Wang, Siqi,Karpov, Victor M.,Mezhenkova, Tatyana V.
, (2021/07/28)
In the reactions with aqueous K2CO3, per...
363-72-4 Process route
-
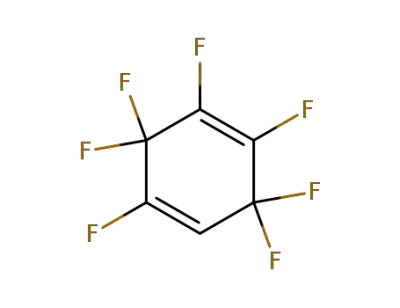
-
773-53-5
2,3,3,4,5,6,6-heptafluorocyclohexa-1,4-diene

-
-
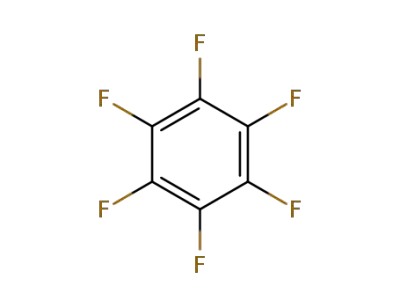
392-56-3
Hexafluorobenzene

-
-
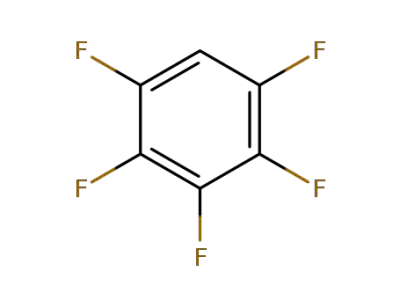
363-72-4
Pentafluorobenzene

-
-
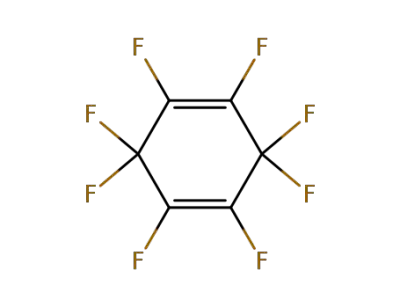
775-51-9
octafluorocyclohexa-1,4-diene

-
-
1998-56-7
2H-heptafluorocyclohexa-1,3-diene

-
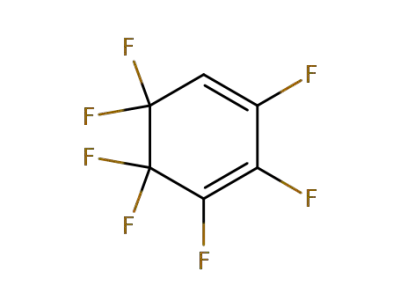
-
878-43-3
1H-heptafluorocyclohexa-1,3-diene

-
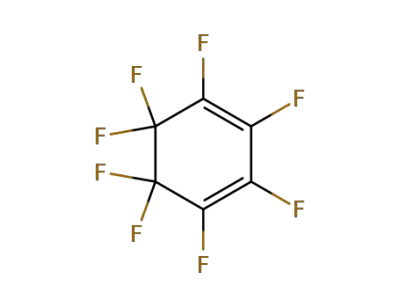
-
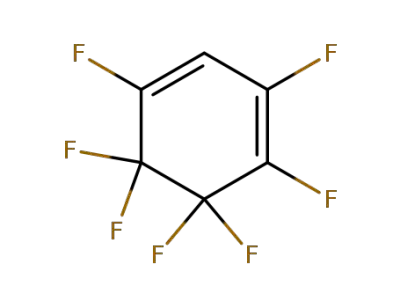
377-70-8
perfluoro-1,3-cyclohexadiene
| Conditions | Yield |
|---|---|
|
With
silica wool;
In
gaseous matrix;
at 700 ℃;
under 10 Torr;
Product distribution;
Mechanism;
thermal isomerization at flash pyrolysis; variation of temperature and pressure;
|
0.5 % Chromat. 1.7 % Chromat. 12.8 % Chromat. 6.6 % Chromat. 28.6 % Chromat. 11.6 % Chromat. |
-

-
1998-56-7
2H-heptafluorocyclohexa-1,3-diene

-

-
392-56-3
Hexafluorobenzene

-

-
363-72-4
Pentafluorobenzene

-

-
773-53-5
2,3,3,4,5,6,6-heptafluorocyclohexa-1,4-diene

-

-
775-51-9
octafluorocyclohexa-1,4-diene

-

-
878-43-3
1H-heptafluorocyclohexa-1,3-diene

-

-
377-70-8
perfluoro-1,3-cyclohexadiene
| Conditions | Yield |
|---|---|
|
With
silica wool;
In
gaseous matrix;
at 700 ℃;
under 0.001 Torr;
Product distribution;
Mechanism;
thermal isomerization at flash pyrolysis; variation of temperature and pressure;
|
0.3 % Chromat. 3.8 % Chromat. 19.2 % Chromat. 13.1 % Chromat. 35.4 % Chromat. 12.8 % Chromat. |
363-72-4 Upstream products
-
344-04-7
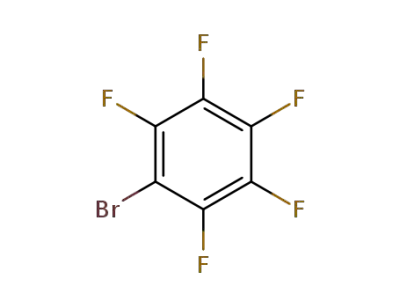
bromopentafluorobenzene
-
878-43-3

1H-heptafluorocyclohexa-1,3-diene
-
110-89-4
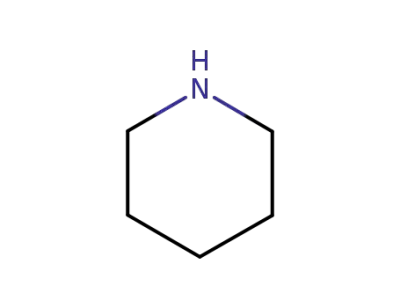
piperidine
-
1206-46-8
trimethyl(pentafluorophenyl)silane
363-72-4 Downstream products
-
2367-82-0
1,2,3,5-tetrafluorobenzene
-
344-04-7

bromopentafluorobenzene
-
2324-98-3
2,3,5,6-tetrafluoroanisole
-
827-15-6
1,2,3,4,5-pentafluoro-6-iodobenzene
Relevant Products
-
5-Bromo-2-fluoro-m-xylene
CAS:99725-44-7
-
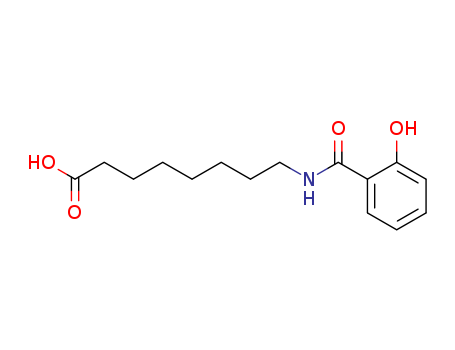
8-[(2-hydroxybenzoyl)amino]octanoic acid
CAS:183990-46-7
-
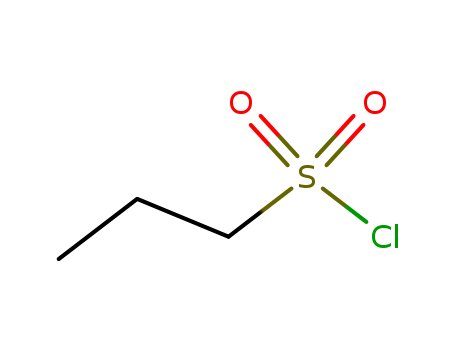
Propanesulphonyl chloride
CAS:10147-36-1